주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
|
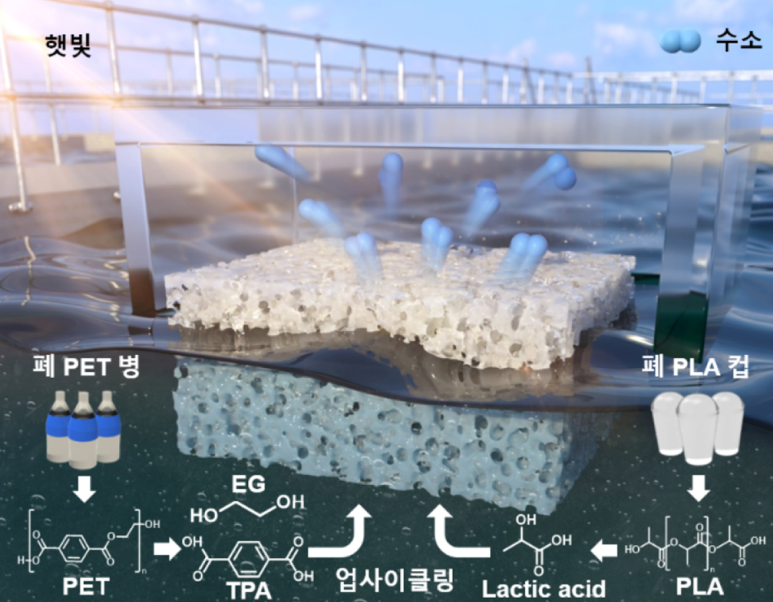
[Science Knowledge Encyclopedia] Experimental Process Uses Sunlight to Extract Hydrogen Fuel from Waste PlasticsMicroplastics that enter the ocean are absorbed by marine organisms and move up the food chain, potentially affecting the human diet. Concerns about health impacts and biodiversity loss linked to this process have been raised internationally. The United Nations Environment Programme (UNEP) and environmental authorities in many countries have designated plastic pollution as a global emergency, emphasizing the urgent need for technological solutions. Meanwhile, hydrogen is being promoted as a next-generation energy carrier to replace fossil fuels. Because it does not release carbon dioxide when used and can serve as a source of power and heat in many industries, hydrogen is positioned as a key energy option in carbon-neutral strategies. However, about 95 percent of hydrogen worldwide is still produced from fossil fuels such as natural gas through high-temperature, high-pressure processes, which result in substantial greenhouse gas emissions. Against this background, demand is growing for so-called “green hydrogen” technologies that can generate hydrogen using natural resources such as sunlight or water rather than fossil fuels. Hydrogen production systems coupled with renewable energy sources are receiving strong attention from governments and industry worldwide as a strategic pathway to carbon neutrality. At the intersection of the plastic pollution challenge and the search for low-carbon energy, technologies that convert plastics into hydrogen are being presented as more than simple technical innovations. Advocates describe them as integrated approaches that could simultaneously address environmental problems and provide new energy resources. A Floating Catalyst: A New Solution for Hydrogen ProductionThe research team has developed an environmentally friendly and highly efficient photocatalyst system capable of converting waste plastics into hydrogen using only sunlight. The most distinctive feature of this system is its design that overcomes the structural limitations of conventional catalysts. To ensure stable reactions under real-world conditions, the photocatalyst particles are encapsulated in a polymer hydrogel that allows them to float on the water’s surface. This “floating structure” not only maximizes light absorption efficiency in photocatalytic reactions but also facilitates the smooth release of the generated gases from the surface. Most existing photocatalyst systems sink and operate underwater, where light penetration is limited, the transport of reactants is slow, and the generated gases can drive undesirable reverse reactions. In contrast, the new system operates directly at the air–water interface, thereby addressing these problems. In particular, the polymer hydrogel network evenly disperses and secures the catalyst particles while preventing detachment or damage under strongly alkaline conditions. This structure is designed to maintain reaction stability over extended periods and possesses durability against external shocks and physical disturbances. Experimental results showed that the system maintained stable hydrogen production for more than two months, even in strongly alkaline environments with pH values above 14. It also demonstrated consistent reactivity under various water conditions, including seawater, tap water, and rainwater. These findings suggest that the technology could be applied in practical environments such as wastewater treatment plants, marine structures, and municipal water systems.
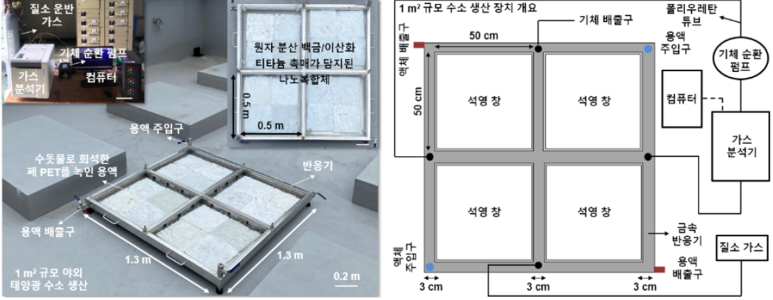
Figure 1. Hydrogen production from waste plastics using sunlight The research team enhanced both stability and activity by firmly anchoring the catalyst within a polymer network and positioning it at the gas–liquid interface. They fabricated a nanocomposite in which atomically dispersed platinum/titanium dioxide catalysts were uniformly attached to a sponge-like, lightweight polymer hydrogel. This floating structure was then placed at the interface of solutions derived from decomposed PET bottles and PLA cups, where it produced hydrogen under sunlight. The polymer network chemically and physically bound the catalyst securely, enabling stable operation at high performance for more than two months even under harsh alkaline conditions. Durability and Scalability Demonstrated in Outdoor ExperimentsTo verify the practicality of the system, the research team designed an outdoor reaction device with a surface area of 1 m² and carried out hydrogen production experiments under real sunlight. Within the steel reactor, the floating photocatalysts were installed, and the hydrogen generated from PET waste solutions was carried along with nitrogen carrier gas to the analysis equipment. The system stably produced up to about 0.9 liters of hydrogen per day and maintained consistent operation without any decline in catalytic performance or physical damage during the test period. During this time, the team also collected real-time meteorological data—such as solar intensity, temperature, and humidity—alongside hydrogen production records, enabling evaluation of the system’s adaptability to different seasonal and climatic conditions. These results show that the catalyst system is not limited to controlled laboratory environments but can also guarantee sufficient durability and performance in real outdoor settings. Furthermore, because the catalyst modules are small and modular, they can potentially be scaled up into 10–100 m² reactors in the future, with possible applications ranging from residential power supply to industrial energy self-sufficiency.
Figure 2. Large-area (1 m²) installation confirms potential for industrial application The research team scaled the technology up to a 1 m² outdoor installation. By arranging the nanocomposites containing atomically dispersed platinum/titanium dioxide catalysts and connecting them to gas analysis equipment, they produced hydrogen from plastic solutions using only sunlight. The system stably generated hydrogen for over a month, demonstrating the durability and scalability required for industrial applications. Potential for Hydrogen Production from PlasticsThis study presents a technological possibility for converting commonly discarded plastics such as PET and PLA into hydrogen as well as other chemical products, including terephthalic acid and ethylene glycol, rather than simply treating or incinerating them. The research team expects that this approach could be explored further across different scales and applications. They also demonstrated through experiments that the system can operate stably for extended periods using only sunlight as the energy source. |
| before |
|---|
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20










![[Science Knowledge Encyclopedia] Experimental Process Uses Sunlight to Extract Hydrogen Fuel from Waste Plastics](https://www.ibs.re.kr/dext5data/2025/08/20250820_112456329_17449.jpg)