주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Center for Trapped Ion Quantum Science
- Chemistry
- Life Sciences
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Center for Trapped Ion Quantum Science
- Chemistry
- Life Sciences
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
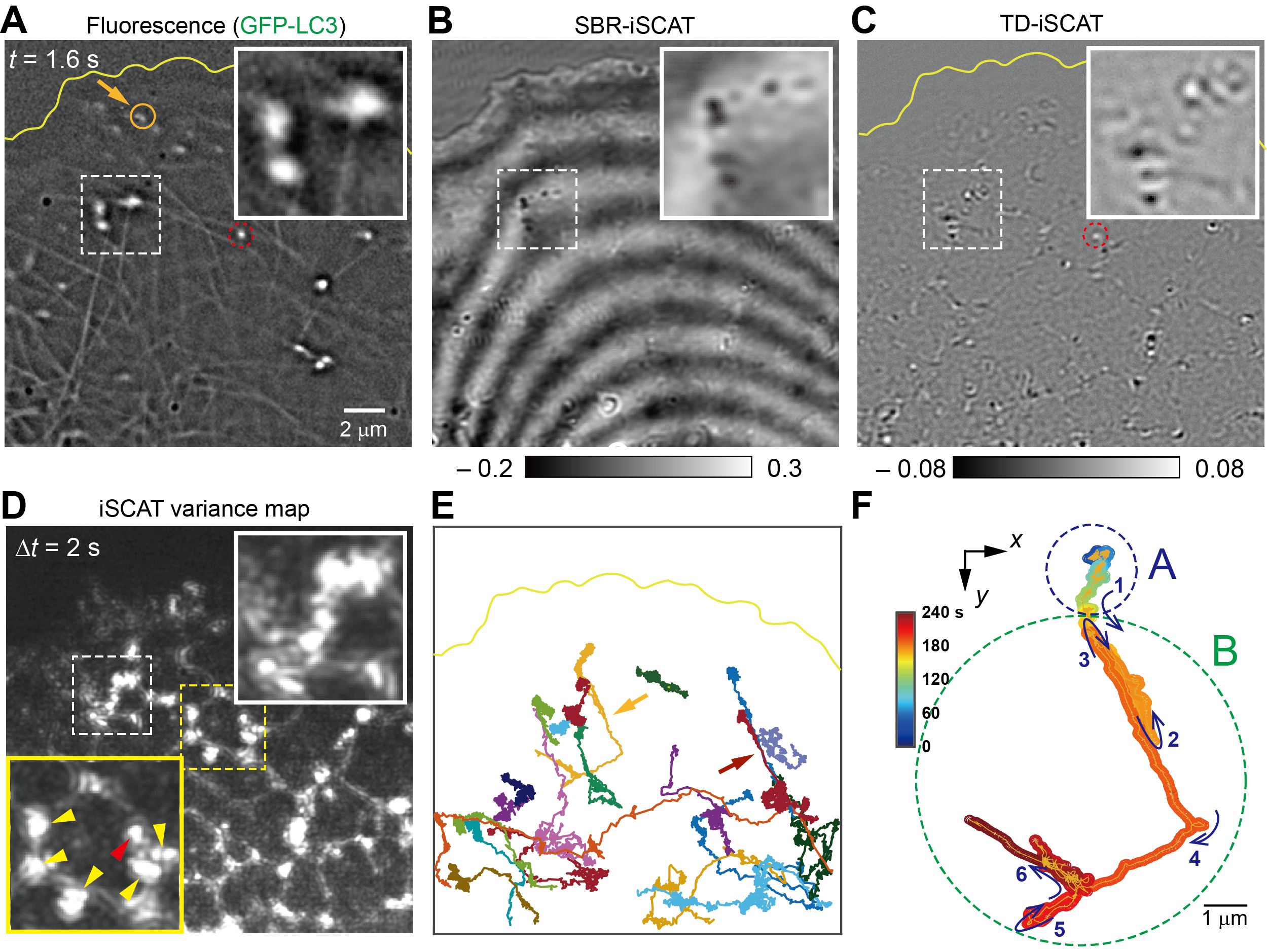
Capturing the Moment of Organelle Handoff Inside Living Cells- Label-free Microscopy Reveals Endoplasmic Reticulum Actively Guides Organelle Transport Toward Microtubules - For the first time, researchers have directly visualized how newly formed cellular organelles leave the endoplasmic reticulum (ER) and transition onto microtubule tracks inside living cells. This new finding reveals that the ER plays an active and dynamic role in steering intracellular traffic rather than serving as a passive factory. In a study led by Director CHO Minhaeng at the Center for Molecular Spectroscopy and Dynamics within the Institute for Basic Science and Professor HONG Seok-Cheol at Korea University, the research team captured in real time the moment an autophagosome—an organelle responsible for cellular recycling—moves from the ER onto a neighboring microtubule. This long-sought observation provides direct experimental evidence for how intracellular transport is coordinated at nanoscopic contact sites within the crowded environment of living cells. Autophagy is an essential cellular process in which damaged proteins and aged organelles are enclosed by double-membrane structures and delivered for degradation and recycling. The importance of autophagy was recognized by the 2016 Nobel Prize in Physiology or Medicine awarded to Yoshinori OHSUMI. Although scientists have long proposed that autophagosomes are transferred from the ER to microtubules at specialized contact sites, direct real-time experimental evidence of this cellular “handoff” had remained out of reach—until now. To overcome this challenge, the researchers combined interferometric scattering microscopy with fluorescence imaging in a custom-built platform. The team fluorescently labeled LC3, a protein essential for autophagosome formation. Because LC3 is also associated with microtubules, the single fluorescent label allowed the researchers to visualize both autophagosomes and microtubules simultaneously. In parallel, they employed DySLIM (Dynamic Scattering-particle Localization Interference Microscopy), a label-free imaging technique based on interferometric scattering microscopy, to capture the ER network and its rapid structural remodeling. This allowed the researchers to achieve millisecond-scale temporal resolution and nanometer-scale spatial precision together with chemical specificity to autophagosomes and microtubule networks. The observations revealed that organelles emerging from the ER do not simply encounter microtubules at random. Instead, they undergo biased diffusion along ER tubules, gradually drifting toward nearby microtubule filaments. Critical to this process are ER three-way junctions—nanoscopic branching points in the ER network—which function as temporary hubs where organelles pause, interact with multiple microtubules, and then launch onto a selected track for long-range transport. Remarkably, the ER remains physically tethered to the departing organelle even after it engages the microtubule. As the organelle moves away, it pulls and extends ER tubules, forming new branches and junctions. In this way, organelle export is coupled to continuous ER remodeling, enabling the ER network to dynamically adapt to ongoing transport demands. When the team disrupted the microtubule network pharmacologically, long-range transport events disappeared. Organelles instead became trapped or diffusively confined, and the ER network appeared loosened and less structured. These results confirm that microtubules are essential not only for organelle transport but also for maintaining ER architecture through cargo-driven remodeling. “By directly observing organelle interactions in living cells with high-speed, high-sensitivity imaging, this work opens a new research paradigm in which cellular recycling processes can be analyzed in space and time simultaneously,” said Professor HONG Seok-Cheol. Director CHO Minhaeng added, “This study demonstrates the power of label-free, high-speed nanoscopic imaging technologies. By further integrating these approaches, we aim to develop next-generation imaging platforms that move beyond conventional fluorescent labeling.” Published in ACS Nano on January 20, 2026, the study underscores the active role of the endoplasmic reticulum in organizing intracellular transport and reshaping itself in response to cellular traffic demands.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS) |
| before |
|---|
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20