주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
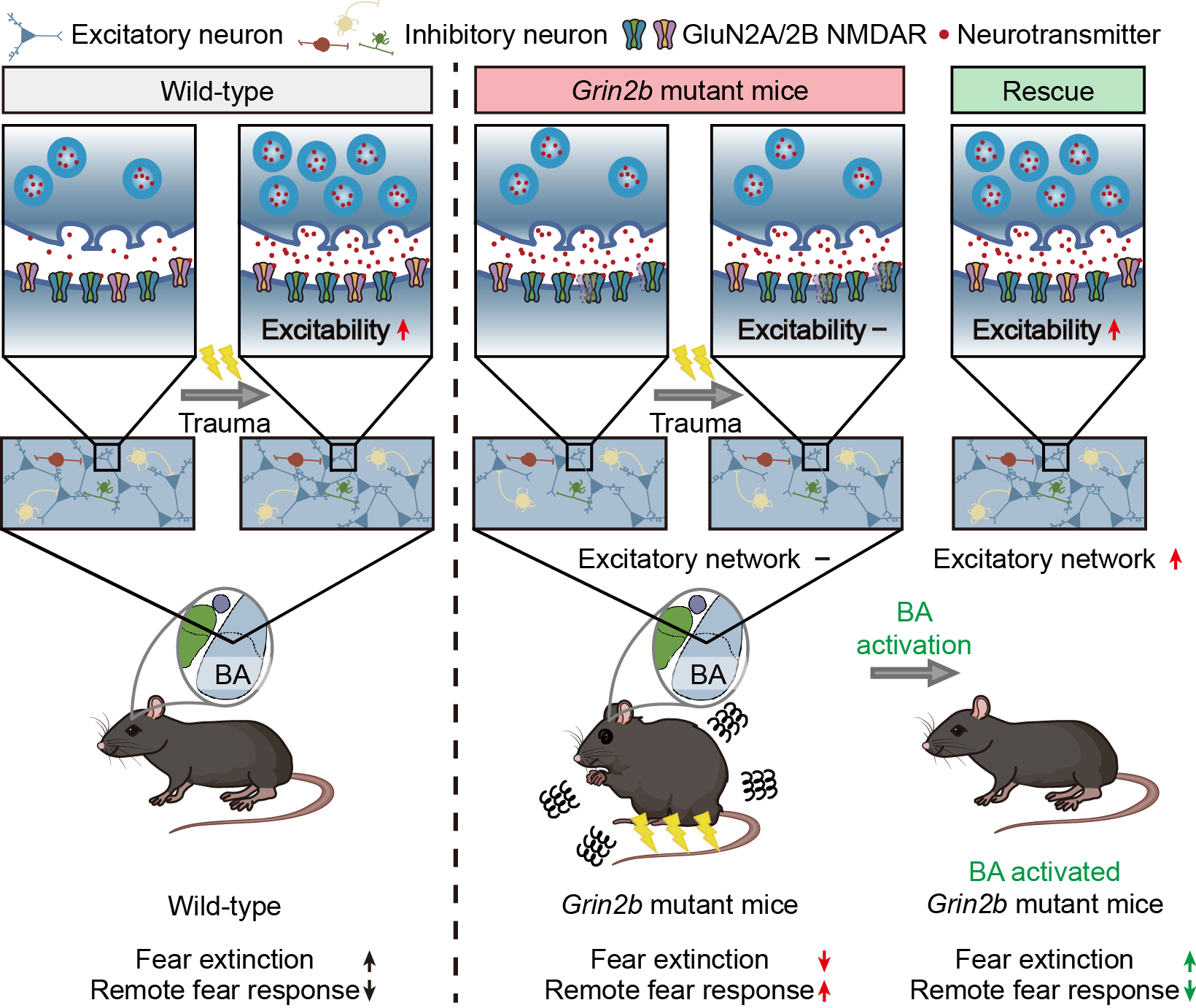
Researchers Reveal How Autism-Linked Mutation Triggers PTSD-like Fear- Discovery of disrupted amygdala circuits in mouse model explains heightened trauma vulnerability in autism - Autism spectrum disorder (ASD) is widely known for its core features, which include difficulties in social communication and repetitive behaviors. But beyond these, many individuals with ASD also struggle with comorbid conditions, particularly anxiety. Nearly 40 percent of children with ASD experience anxiety disorders and often show unusually heightened fear responses. Studies have even suggested that people with ASD may be more vulnerable to trauma, unable to “erase” fear memories, which resembles symptoms seen in post-traumatic stress disorder (PTSD). Until now, most evidence for PTSD-like symptoms in ASD has relied on self-reports, leaving the underlying brain mechanisms unclear. In a new study, researchers led by Professor KIM Eunjoon at the Institute for Basic Science (IBS) have uncovered how an ASD-related mutation in the Grin2b gene, which encodes the GluN2B subunit of NMDA receptors, disrupts brain circuits that normally extinguish fear. Their findings, published in Science Advances, provide the first detailed mechanistic explanation for why ASD increases PTSD risk. The team used mice carrying a human ASD-linked mutation in Grin2b. These mice learned fear memories normally after a traumatic experience, but were unable to extinguish them. As a result, they displayed exaggerated long-term fear responses — closely resembling PTSD. Brain mapping revealed that the basal amygdala (BA), a key hub for fear-memory extinction, became abnormally silent after trauma in mutant mice. “These animals could learn fear just fine, but they couldn’t unlearn it,” said Kim. “The amygdala essentially shut down when it was needed most, leaving the traumatic memory locked in place.” Electrophysiological studies showed that after trauma, BA excitatory neurons in mutant mice exhibited suppressed synaptic activity and reduced excitability. This dysfunction prevented the brain from erasing fear. To test whether this silencing caused the PTSD-like behavior, the team used chemogenetic tools to artificially reactivate BA neurons during extinction training. Remarkably, this restored excitatory signaling, normalized neuronal activity, and rescued both fear-memory extinction and long-term fear responses. “These results show that the silenced amygdala after trauma is the root of PTSD-like symptoms in our model,” explained co-first author KANG Muwon. “By reactivating those neurons, we could reverse the behavioral and physiological abnormalities.” The researchers took care to ensure that viral tools used to manipulate neurons did not themselves interfere with results. They carefully calibrated doses and repeated experiments to confirm reliability. Interdisciplinary collaboration with other groups also helped pinpoint the basal amygdala as the disease-relevant brain region. This study is the first to establish the molecular and circuit-level mechanisms underlying PTSD-like symptoms in autism. By showing that a mutation in Grin2b silences amygdala neurons after trauma — and that reactivation can reverse this effect — the research provides valuable clues for future therapies. “This work bridges a crucial gap,” said Kim. “We move from anecdotal reports of trauma vulnerability in autism to a clear mechanistic understanding of why it happens, and even show that it can be reversed in a model system.” The team plans to extend their work by combining transcriptomic and proteomic analyses to examine gene expression changes in BA excitatory neurons after trauma. They also intend to test receptor-specific agonists and antagonists targeting GluN2B-containing NMDA receptors, which may further illuminate therapeutic pathways.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS) |
| 이전 |
|---|
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20