주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
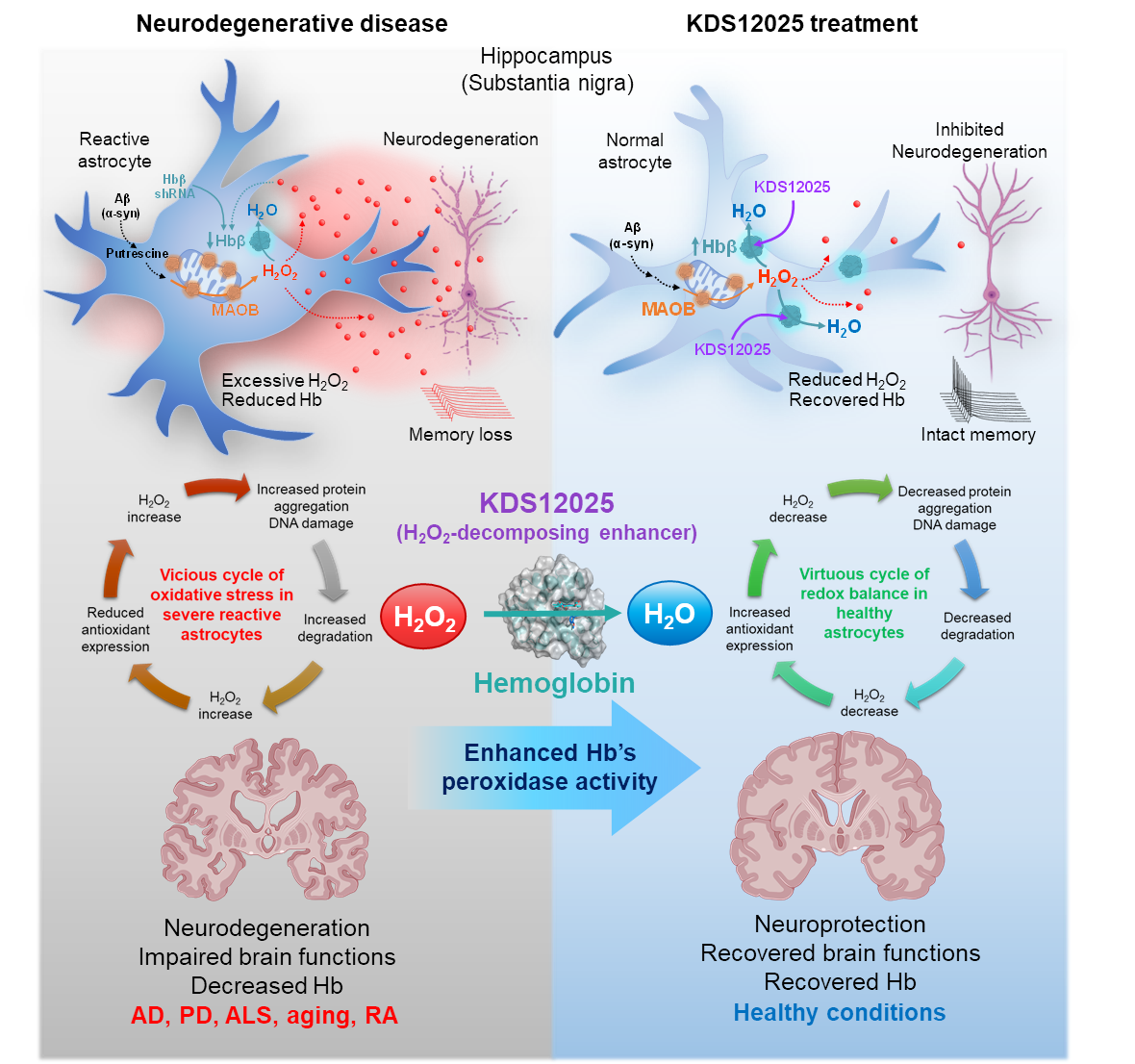
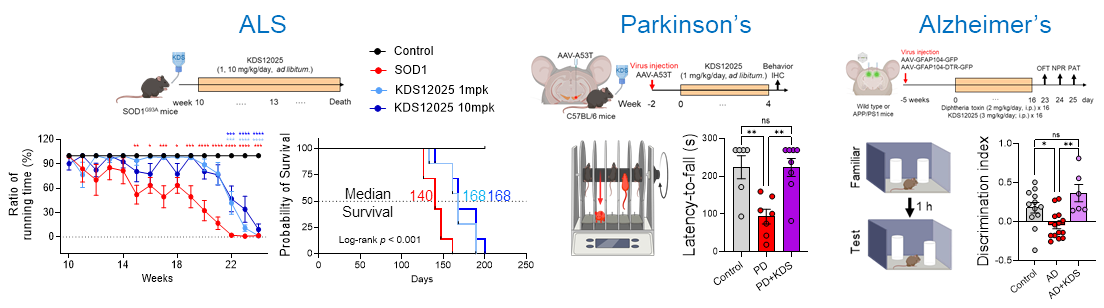
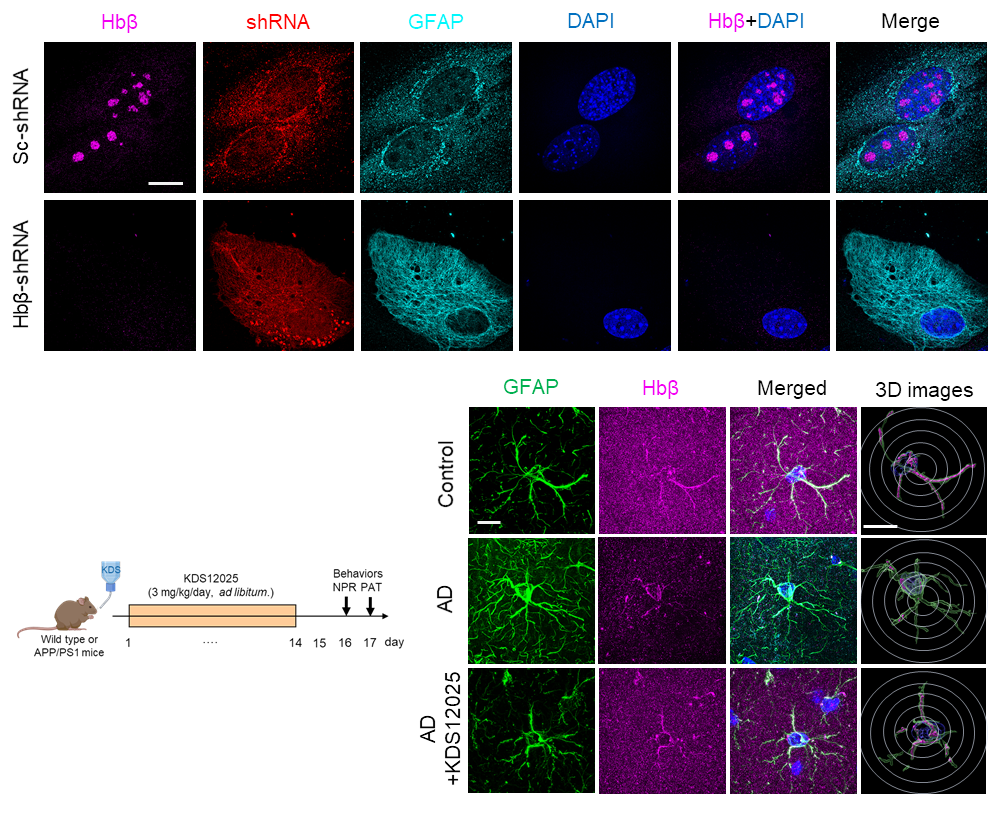
Hemoglobin Reimagined: A Breakthrough in Brain Disease Treatment– Researchers uncover hemoglobin’s antioxidant role in brain cells and boost it to fight ALS, Parkinson’s, Alzheimer’s, aging, and autoimmune disorders – Did you know the same protein that gives blood its red color and carries oxygen throughout the body is also present inside brain cells? Hemoglobin, long celebrated for ferrying oxygen in red blood cells, has now been revealed to play an overlooked — and potentially game-changing — antioxidant role in the brain. In neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Parkinson’s, Alzheimer’s, and aging, brain cells endure relentless damage from the aberrant (or excessive) reactive oxygen species (ROS). For decades, scientists have tried to neutralize ROS with antioxidant drugs, but most failed: they couldn’t penetrate the brain effectively, unstable, or indiscriminately damaged healthy cells. This new study, led by Director C. Justin LEE of the Center for Cognition and Sociality within the Institute for Basic Science (IBS) in Daejeon, South Korea, set out to identify the brain’s own defenses against a particularly harmful form of ROS — hydrogen peroxide (H₂O₂). Using advanced imaging and molecular analysis, the team discovered that hemoglobin exists in the nucleolus of astrocytes — star-shaped brain cells critical for neuronal support — where it acts as a “pseudoperoxidase,” breaking down H₂O₂ into harmless water. “The key was to uncover hemoglobin’s antioxidant potential in the brain and design a ‘first-in-class’ compound that could selectively enhance it. By boosting a natural defense mechanism rather than introducing an external antioxidant, we achieved strong and lasting protection across multiple disease models associated with oxidative stress,” said first author Dr. WON Woojin. The team developed KDS12025, a small, water-soluble molecule capable of crossing the blood–brain barrier. KDS12025 binds to hemoglobin’s heme center and boosts its ability to decompose H2O2 by nearly 100-fold, without disrupting its normal oxygen-carrying function. In disease-like conditions in astrocytes, KDS12025 sharply reduced harmful H2O2 levels. In mouse models, oral administration through drinking water protected neurons, calmed reactive astrocytes, and restored brain function. In animal models, oral administration through drinking water suppressed neuronal death, normalized reactive astrocytes, and restored brain function. ALS model mice showed a delayed disease onset and lived more than four weeks longer than untreated controls. In Parkinson’s models, KDS12025 restored motor function, while in Alzheimer’s models, it recovered memory performance. In aging mice, the treatment extended median lifespan from the typical two years to as long as three years. The drug also alleviated inflammation and joint damage in a rheumatoid arthritis model. The study also uncovered a damaging feedback loop: excess H2O2 depletes astrocytic hemoglobin, weakening the brain’s natural antioxidant defenses and accelerating degeneration. By boosting hemoglobin’s levels and activity, KDS12025 reversed this trend, reducing oxidative stress, preserving neurons, and maintaining healthy brain function. No previous treatment has targeted astrocytic hemoglobin as an antioxidant system, nor demonstrated such broad protective effects. “This is a major step forward in the fight against neurodegenerative diseases. By enhancing the brain’s own hemoglobin to combat oxidative stress, we are opening an entirely new therapeutic avenue,” said Director Lee. The team now plans to further study the distinct roles of α- and β-globin in the brain, refine KDS12025 derivatives for potential human use, and explore its applications in other oxidative stress–driven disorders. This work transforms hemoglobin from a familiar oxygen transporter into a precision antioxidant defense system for the brain. It marks a potential paradigm shift in how scientists approach neurodegenerative diseases, age-related decline, and autoimmune conditions.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS) |
| before |
|---|
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20