주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
Exploring the frontiers of synaptic research with genetically encoded fluorescent tools- This review elaborates on conceptual bases in synapse labelling techniques and their broader implications for neuroscience research - A seminal review highlighting recent advances in genetically encoded fluorescent tools for labeling and selectively manipulating synapses has been published in Nature Reviews Neuroscience. This innovative review is led by Director KAANG Bong-Kiun, Research Fellow LEE Sangkyu, and Postdoctoral Researcher Binod TIMALSINA from the Center for Cognition and Sociality (CCS) at the Institute for Basic Science (IBS) in Daejeon, South Korea. Director KAANG is a pioneering scientist who has made significant contributions to studying how synapses work, especially in how they relate to learning and memory. The authors have elaborated the cutting-edge genetically encoded tools and how they can be used to label and selectively manipulate the synapses. Synapses, the junctions between neurons, play a crucial role in transmitting signals within the brain. Beyond their basic function of signal transmission, synapses are key to essential processes like learning, memory formation, and brain adaptation. Dysfunction in synaptic activity is linked to various neurological conditions, including Alzheimer’s disease, epilepsy, and autism spectrum disorders, making the study of synapses crucial for understanding and potentially treating these disorders. Although several tools for labeling synapses have been reported to date, an ideal tool for selectively labeling and manipulating synapses is still lacking. This review explores synapse labeling approaches by categorizing them according to their conceptual foundations and target molecules. The authors focus on molecular techniques, highlighting their advantages, limitations, and potential applications. They also propose possible advancements for existing tools. Synapse labeling tools are classified into three categories: those targeting synaptic contacts, individual synaptic molecules, and the interface between presynaptic and postsynaptic membranes. The IBS team has been at the forefront of developing several innovative synapse labeling tools, including dual-enhanced green fluorescent protein reconstitution across synaptic partners (dual-eGRASP), Local circuit (LCD-eGRASP), and SynapShot techniques. Dual-eGRASP and LCD-eGRASP can selectively label synapses associated with memory encoding, referred to as 'engram synapses,' and those not involved in memory, or 'non-engram synapses,' using cyan, yellow, and green fluorescent proteins. These tools are extensively used to explore mechanisms of learning and memory in the mouse brain. Recently, there has been growth in tools designed to selectively manipulate synapse function using molecular actuators and optogenetic approaches. This review highlights some of the most promising tools, such as Activated synapse targeting photoactivatable Rac1 (As-PaRac1), SuperNova, and photoactivatable botulinum neurotoxin (PA-BoNT). Professor Kaang states, “This review marks a milestone in synapse studies, showcasing advancements in existing tools that will help us unravel the mysteries of synaptic function and related disorders. We hope our work garners global attention from those in the filed of synapse research.” The authors emphasize novel approaches for designing future tools for synapse labeling and understanding their functions. These advancements aim to provide deeper insights into synaptic plasticity, which is often compromised in neurodegenerative diseases. Additionally, such tools could be instrumental in developing potential therapeutic interventions for neurological disorders related to synaptopathy.
Synapse labeling strategies involve marking synaptic contact sites with different fluorescent proteins in the pre- and postsynaptic compartments. The interface between these contacts is also labeled, and active synapses are manipulated using optogenetic approaches.
Active synapses are labeled with different fluorescent proteins using the dual-eGRASP technique, which targets neural circuits connecting various regions of the mouse brain involved in specific memories. The LCD-eGRASP technique further advances this approach by allowing the study of active synapses within localized brain regions.
The As-PaRac1 technique uses blue light to activate RAC1 protein, leading to actin disassembly and dendritic spine shrinkage in the postsynaptic neuron. Similarly, the Cofilin-SuperNova technique employs a cofilin-targeted modified Killer Red protein that produces reactive oxygen species (ROS) upon light exposure, disrupting F-actin assembly and thereby weakening synaptic interactions. Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20










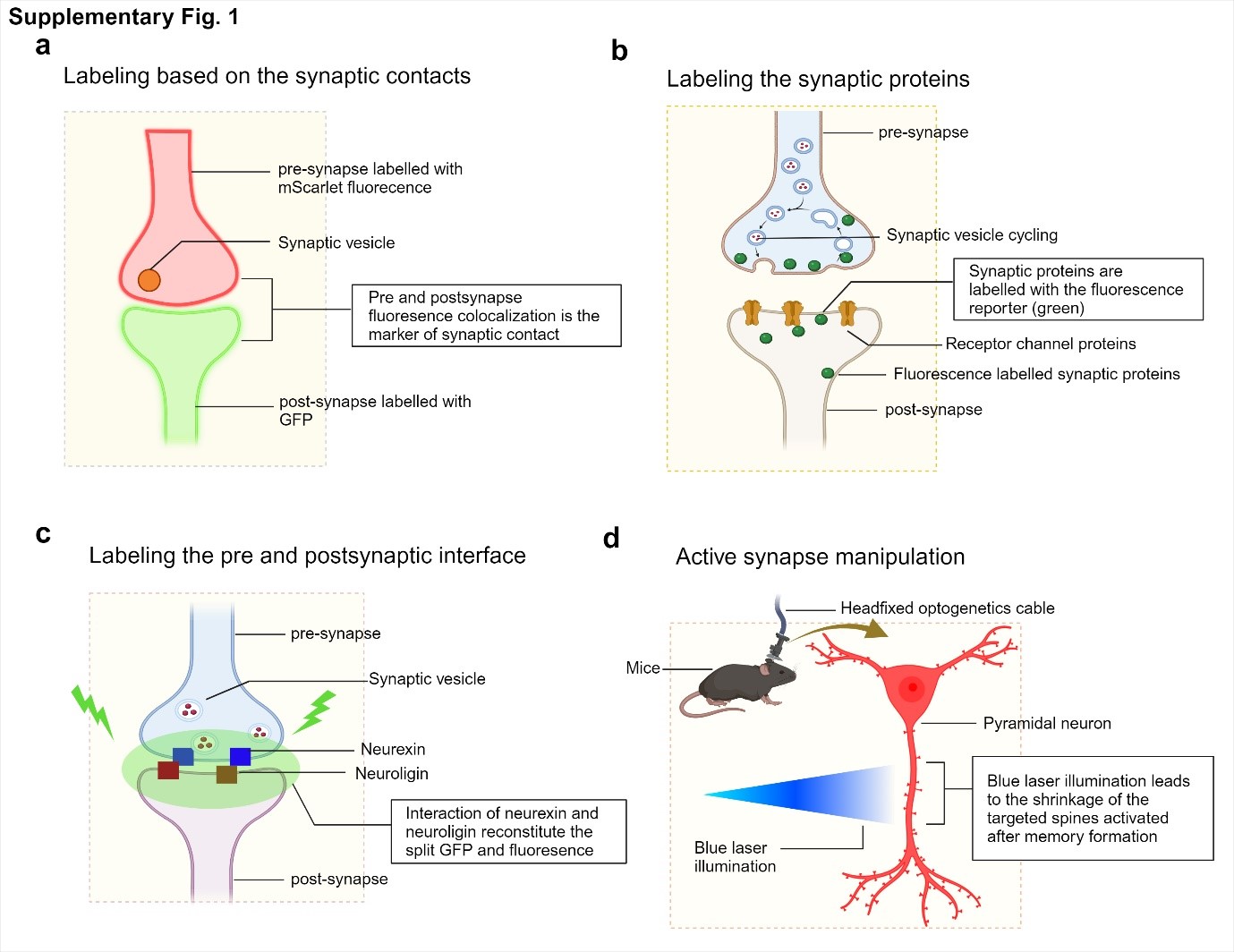 Figure 1. Overview of strategies for synaptic labelling, monitoring, and manipulation
Figure 1. Overview of strategies for synaptic labelling, monitoring, and manipulation
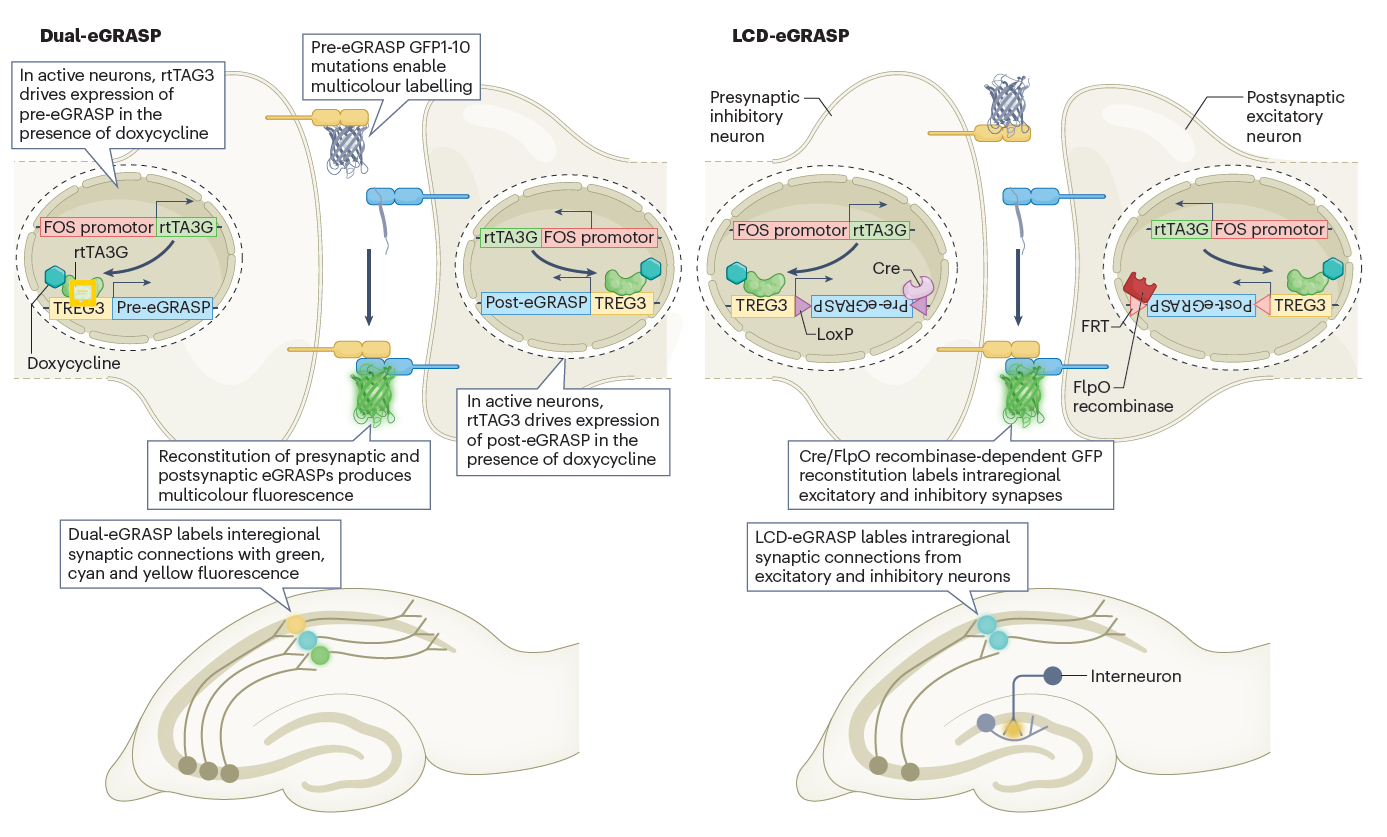 Figure 2. Tools to label the active synapses and trace the neural circuits
Figure 2. Tools to label the active synapses and trace the neural circuits
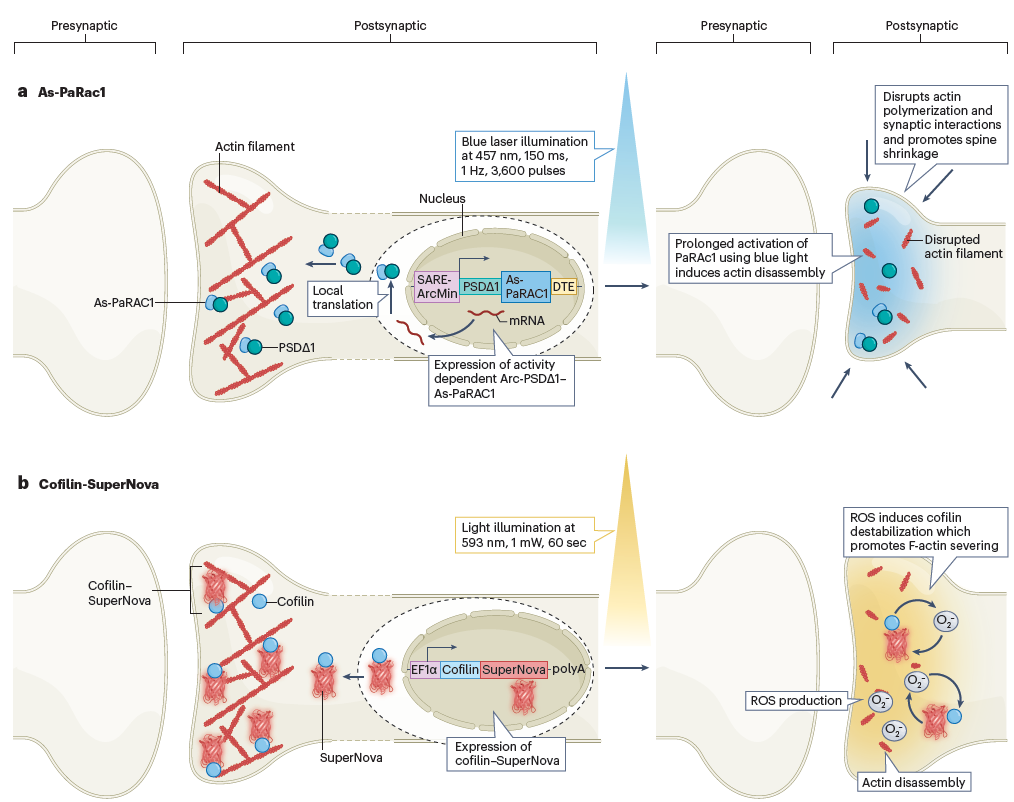 Figure 3. Techniques for silencing active synapses
Figure 3. Techniques for silencing active synapses

