주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
Post Office in Brain Cells: Key Ion Channel that Activates Golgi Apparatus Revealed- Scientists Identify Cation Channel Responsible for Protein Modification and Transport in Cells - The research team led by Director C Justin LEE of the Center for Cognition and Sociality within the Institute for Basic Science (IBS), along with the team led by Chief Investigator KIM Ho-Min of the Center for Biomolecular and Cellular Structure, has elucidated the operational mechanism of a key ion channel crucial for maintaining the shape and function of the Golgi apparatus, an organelle responsible for the modification and transport of proteins within cells. The study also revealed the relationship between damage to this ion channel in brain cells and cognitive impairment, presenting new therapeutic targets for brain diseases. The Golgi apparatus acts like a post office within cells, receiving lipids and proteins synthesized by the endoplasmic reticulum, processing and modifying them, and then transporting them to other cellular organelles or outside the cell. For the Golgi apparatus to maintain its shape and function, it is important for the internal environment to be maintained at a mildly acidic pH of 6.0-6.7 by ion channels. Malfunctions in these ion channels are known to cause structural changes in the Golgi apparatus, which is a condition often found in Alzheimer’s disease patients. However, the relationship between Golgi structure and cognitive impairment was not clear due to a lack of understanding of these ion channels. In a quest to identify major ion channels in the brain cell Golgi apparatus, Director C Justin LEE's team explored various uncharacterized membrane proteins. In 2023, they identified that the membrane protein TMEM87A, which is highly expressed in hippocampal astrocytes and neurons, regulates the acidity inside the Golgi apparatus of brain cells by acting as a cation channel. They named this membrane protein "Golgi-pH-regulating cation channel" or "GolpHCat" to emphasize its physiological role. In collaboration with Chief Investigator KIM Ho Min's team, they used IBS's cryo-electron microscopy (Cryo-EM) to determine the three-dimensional molecular structure of GolpHCat at an ultra-high resolution of 3.1 Ångströms (1/100 million cm). They also conducted electrophysiological experiments and molecular dynamics analyses to reveal the ion movement pathway. It was established that GolpHCat is a voltage-dependent channel that opens in response to voltage changes across the cell membrane. GolpHCat has a structure comprising a lumen domain connected to the cell membrane on the external side and a transmembrane domain with seven helices penetrating the membrane, with a cavity in the center of the transmembrane domain. This cavity is physically blocked by fatty acid chains of phosphatidylethanolamine originating from the Golgi membrane, preventing ion passage. However, when voltage is applied, the third helix of the transmembrane domain acts as a voltage sensor, activating and opening the channel. The negatively charged residues inside the funnel-shaped lumen then attract positively charged sodium (Na), potassium (K), and cesium (Cs) ions into the channel. Thus, GolpHCat serves as a pathway for cations, contributing to the maintenance of Golgi membrane voltage and internal acidity in conjunction with anion channels. To confirm the biological effect that arises due to GolpHCat damage, the research team observed brain cells from GolpHCat-deficient mice. These mice exhibited abnormal structural changes such as the Golgi apparatus fragmenting or swelling. These structural changes disrupted one of the Golgi's main functions, protein glycosylation, leading to impaired learning and memory in the mice. These findings highlight the importance of normal GolpHCat function for cognitive abilities and suggest the potential for targeting GolpHCat in treating cognitive disorders. Chief Investigator KIM Ho Min remarked, "This research is a result of a convergence study incorporating various advanced bio research technologies such as neurobiology, structural biology, molecular dynamics, and glycomics, representing a successful case of collaboration that broke down academic barriers." Director C Justin LEE stated, "We have elucidated how structural and functional changes in the Golgi apparatus are involved in memory. Understanding the molecular mechanisms of the Golgi apparatus could lead to new treatments for cognitive impairments found in various neurodegenerative brain diseases." This research, which discovered a new Golgi ion channel and identified its function, structure, and physiological importance, represents a collaborative success within the IBS Life Science Institute. The study was also conducted in collaboration with Professor CHOI Sun Young from Ewha Womans University's College of Pharmacy (supported by the Ministry of Science and ICT and the National Research Foundation of Korea), and Professor AN Hyun Joo from Chungnam National University's Department of Analytical Science and Technology. The results of the study were published online in the journal "Nature Communications" (IF=14.7) on July 11.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20










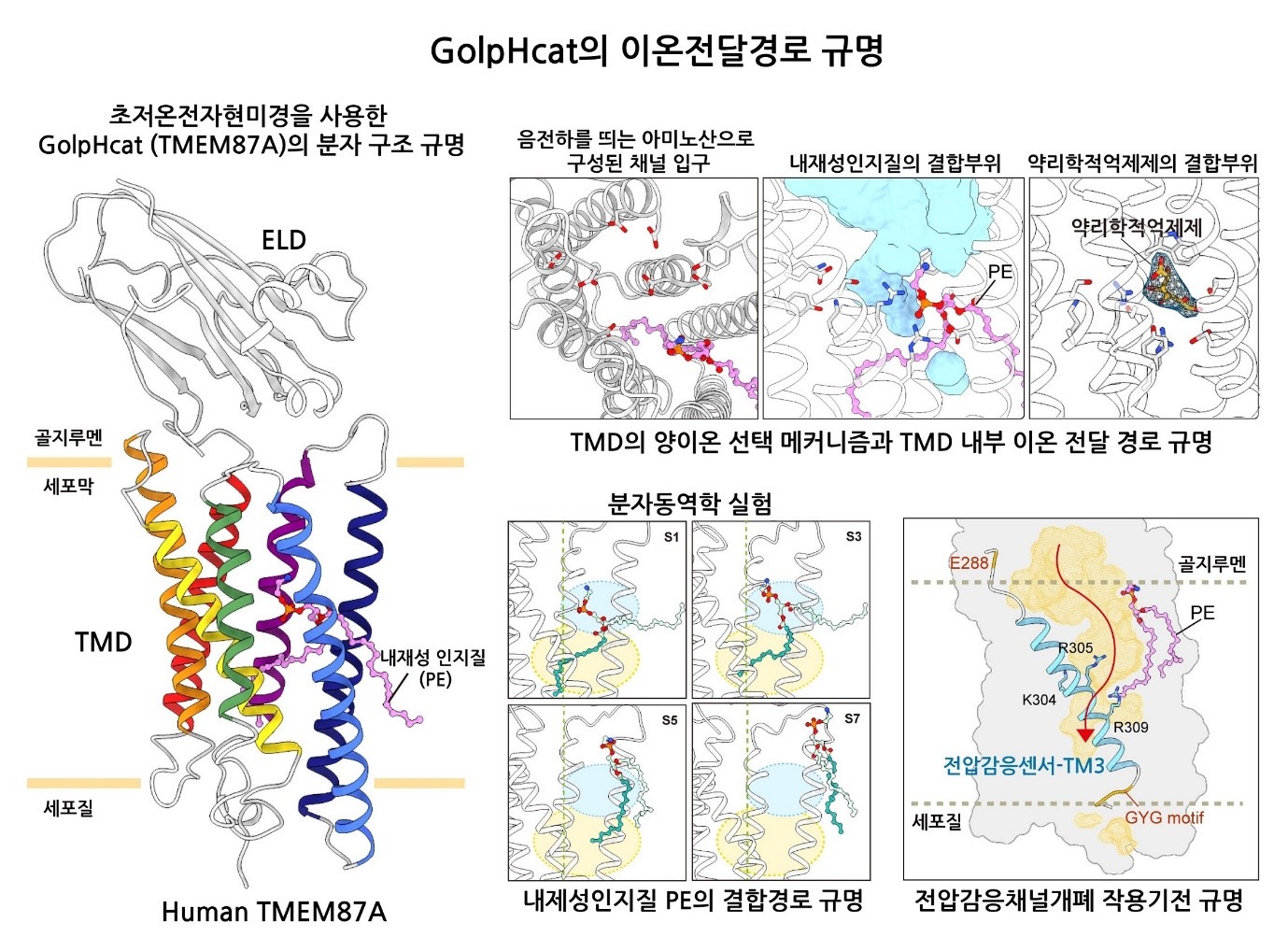 Figure 1. Schematic of Molecular Structure Study of GolpHCat
Figure 1. Schematic of Molecular Structure Study of GolpHCat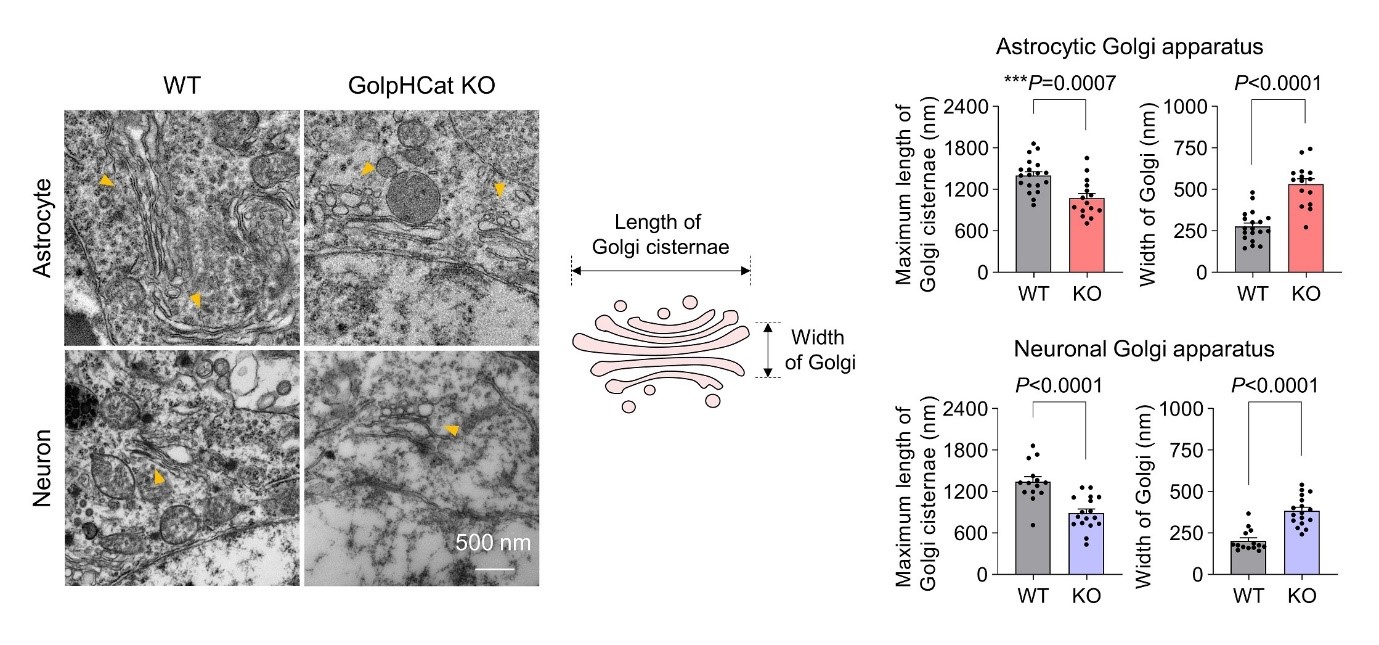 Figure 2. GolpHCat is Important for Maintaining Normal Golgi Structure
Figure 2. GolpHCat is Important for Maintaining Normal Golgi Structure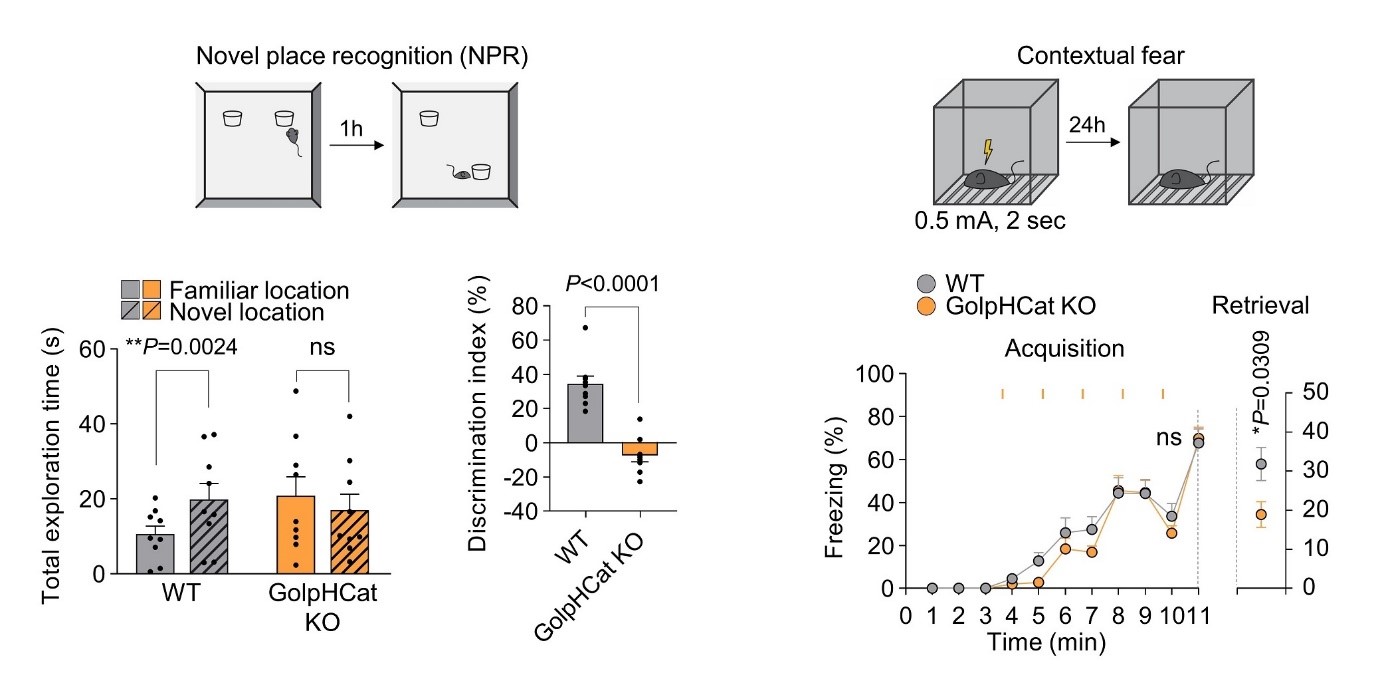 Figure 3. Memory Impairment in GolpHCat Knockout Mice
Figure 3. Memory Impairment in GolpHCat Knockout Mice
