주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
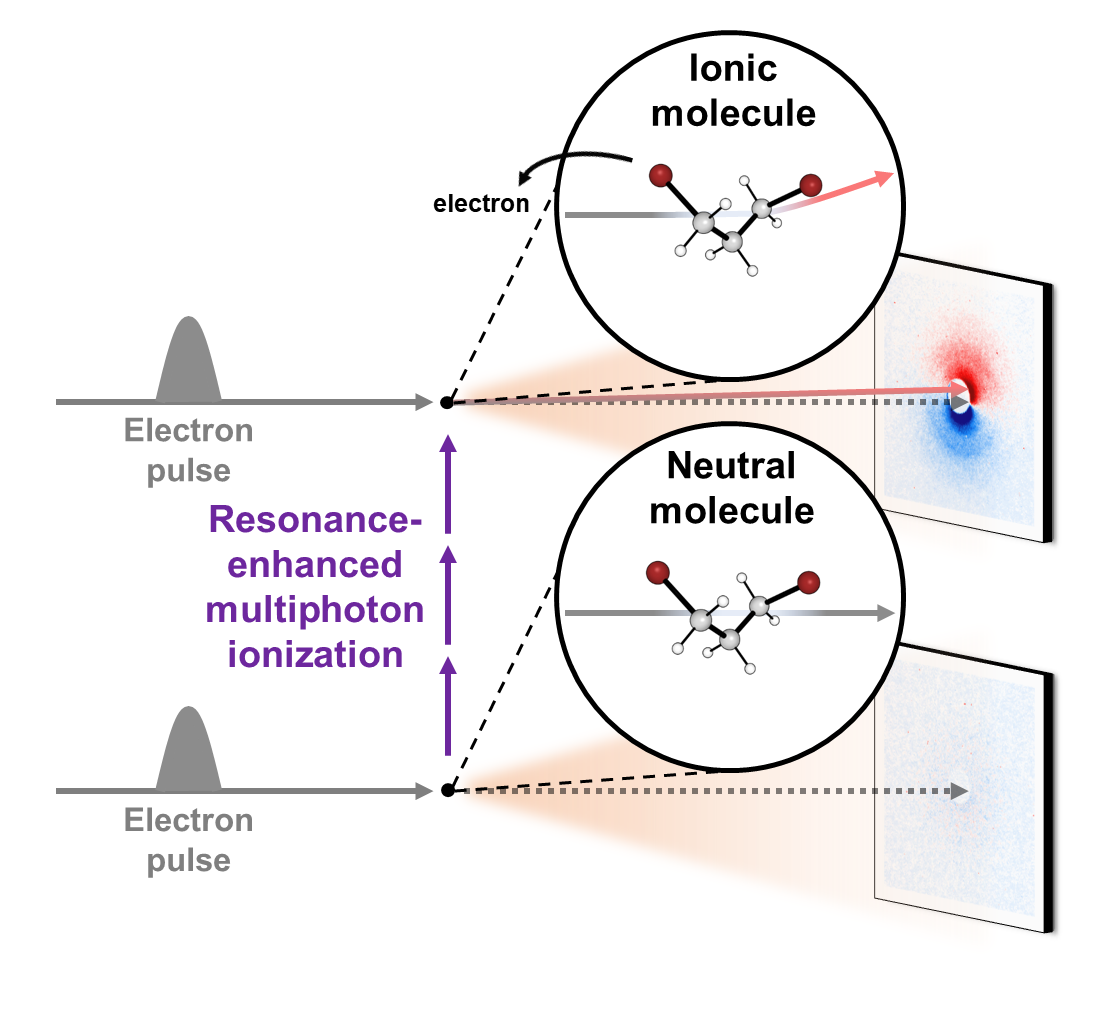
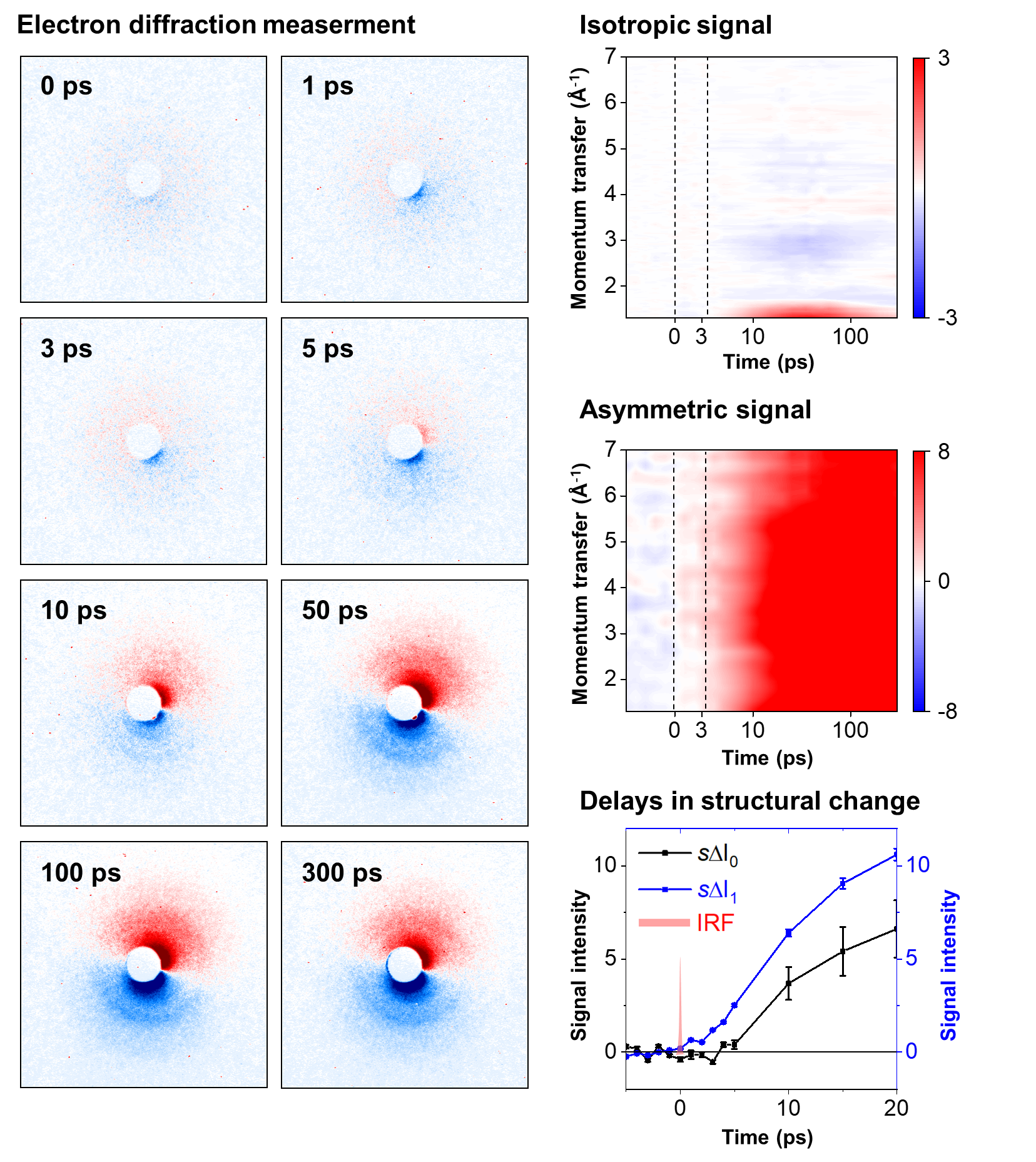
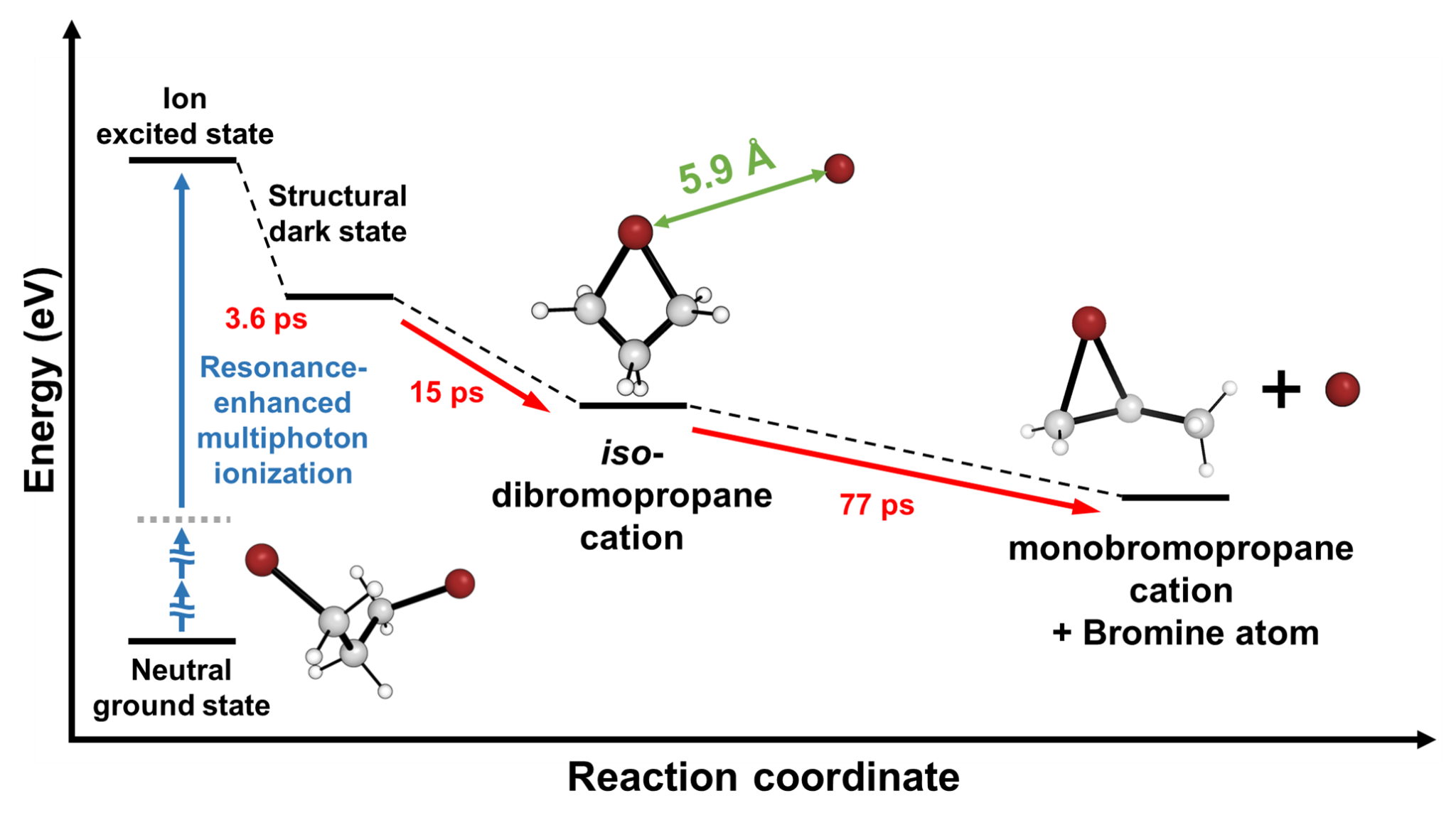
Epic of a molecular ion: with eyes of electrons- Delving into Unexplored Realms, MeV-UED Illuminates the Birth and Structural Evolution of a Molecular Ion - Ions are everywhere, from our daily surroundings to the cosmic expanse. As common table salt (NaCl) dissolves into sodium (Na+) and chloride (Cl-) ions in water, it imparts a salty taste. Once absorbed by the body, these ions regulate nerve impulses and muscle movements. In the sun, plasma—a gathering of ions in the gaseous state—undergoes nuclear fusion reactions, transmitting light and energy to Earth. One of the most noteworthy usage ions in everyday life is found in lithium-ion batteries, which power devices like smartphones, laptops, and electric cars. Consequently, ions play pivotal roles in various facets of our lives, and comprehending the intricate processes, structural attributes, and dynamics of ions remains crucial for advancements in science and technology. However, capturing the ephemeral moments of ion formation and the molecular structural transitions, especially in the challenging gas phase, has proven to be challenging due to experimental complexities Led by Director IHEE Hyotcherl, researchers at the Center for Advanced Reaction Dynamics (CARD) in the Institute for Basic Science (IBS) achieved real-time capture of the ionization process and subsequent structural changes in gas-phase molecules through an enhanced megaelectronvolt ultrafast electron diffraction (MeV-UED) technique, enabling observation of faster and finer movements of ions. Director Ihee’s team had a long history of achieving groundbreaking milestones in molecular dynamics, such as the rupture of molecular bonds (Science, 2005), the initiation of molecular birth through chemical bonding (Nature, 2015), and the in-depth exploration of molecular structures at the atomic level across the entirety of a chemical reaction (Nature, 2020). Now for the first time, they successfully conducted real-time observations of the formation and structural evolution of gas-phase ions. To achieve this objective, the team focused on cations of 1,3-dibromopropane (DBP). Experimental data unveiled a fascinating phenomenon—the cation persisted in a structurally stable state termed the 'dark state' for approximately 3.6 picoseconds (1 picosecond equals one trillionth of a second) following its formation. Subsequently, the cation underwent a transformation into an unusual intermediate with a ring structure encompassing four atoms, including a loosely bound bromine atom. Eventually, the loosely attached bromine atom disengaged, giving rise to a bromonium ion characterized by a ring structure comprising three atoms. Given the high reactivity of ions, observing their existence has posed a longstanding significant challenge. The success of this research hinged on the incorporation of a newly devised signal processing technology and a modeling analysis technique for structural changes. Another important element was the application of the resonance-enhanced multiphoton ionization (REMPI) technique, which facilitated the mass production of specific ions while preventing random dissociation of compounds. The experimental findings indicated that the generated gas ions maintained a specific form before undergoing sudden transformations, which allowed the IBS team to ultimately elucidate the formation of chemically stable, ring-shaped molecules. Then, by leveraging the innovative megaelectronvolt ultrafast electron diffraction (MeV-UED) technique, the research team achieved a precise capture of subtle structural changes in ions within the gas phase. This cutting-edge technology offered high-resolution spatial and temporal resolution required for the needs of this research, and it enabled the meticulous tracking of the entire process from the moment of ion generation to subsequent structural transformations. Being the first to achieve real-time observation of structural changes in selectively generated ions, this study is hailed as a substantial breakthrough in ion chemistry research. This research represents a groundbreaking achievement in the scientific community, marking the inaugural instance of real-time observation of the structural dynamics of molecular ions. By advancing our understanding of ions in the gas phase, this research yields fresh perspectives across diverse fields, including the mechanisms of chemical reactions, alterations in material properties, and the realm of astrochemistry. The anticipated impact extends well beyond ion chemistry, influencing various scientific and technological disciplines. Dr. HEO Jun, the primary author, emphasized, "This discovery represents a pivotal advancement in our fundamental comprehension of ion chemistry, poised to profoundly influence the design of diverse chemical reactions and future exploration in astrochemistry." KIM Doyeong, the first author and a student, shared his aspirations, stating, "Contributing to a study with the potential to lay the groundwork for advancements in basic science is truly gratifying. I am committed to persistent research efforts to evolve into a proficient scientist." Professor IHEE Hyotcherl reflected, "Despite the remarkable strides in science and technology, numerous captivating mysteries remain in the material world. This research, though unveiling just one more enigma of ions previously undiscovered, underscores the profound secrets awaiting our exploration." He further remarked, "The support from the Institute for Basic Science has played a crucial role in achieving this modest yet meaningful milestone. We anticipate sustained, effective backing for R&D budgets in the future."
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20