주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
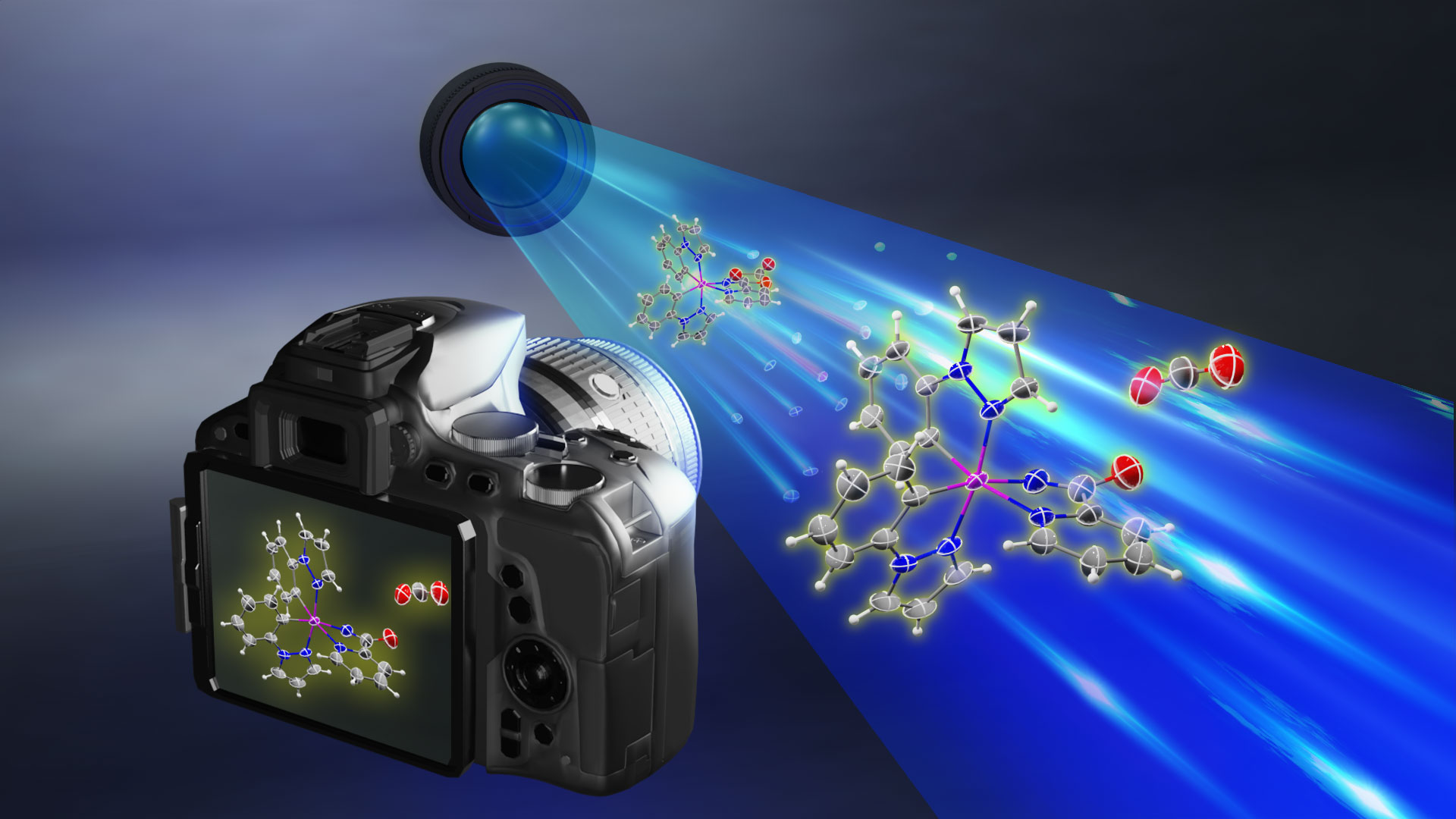
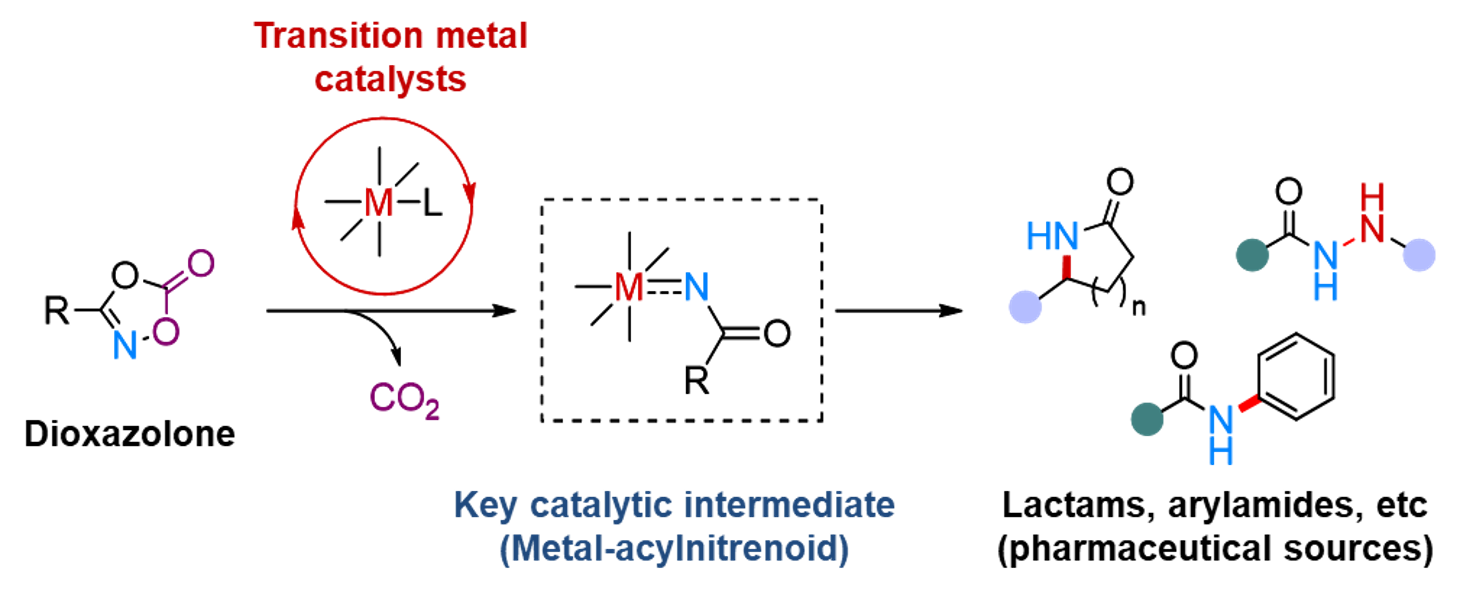
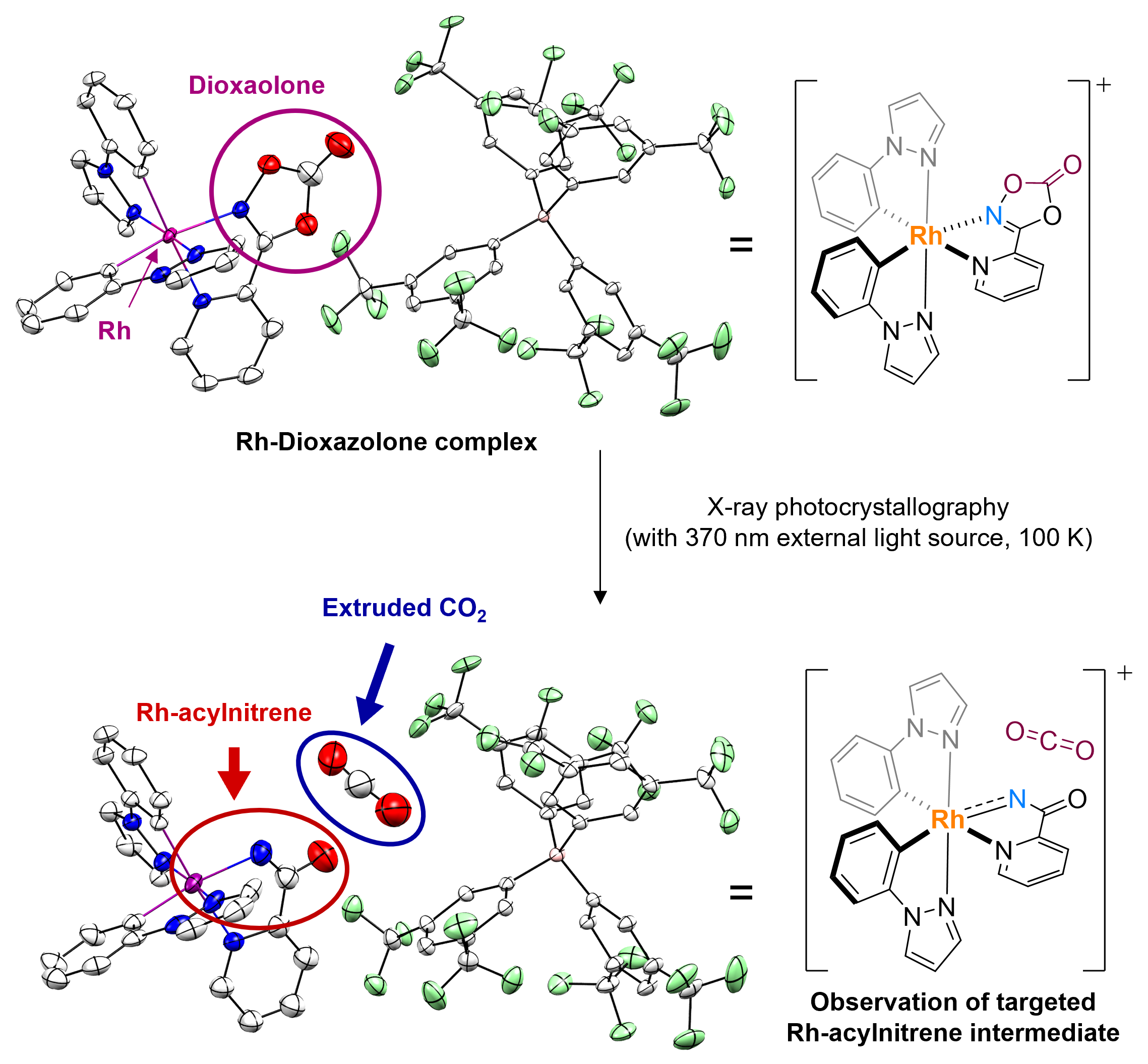
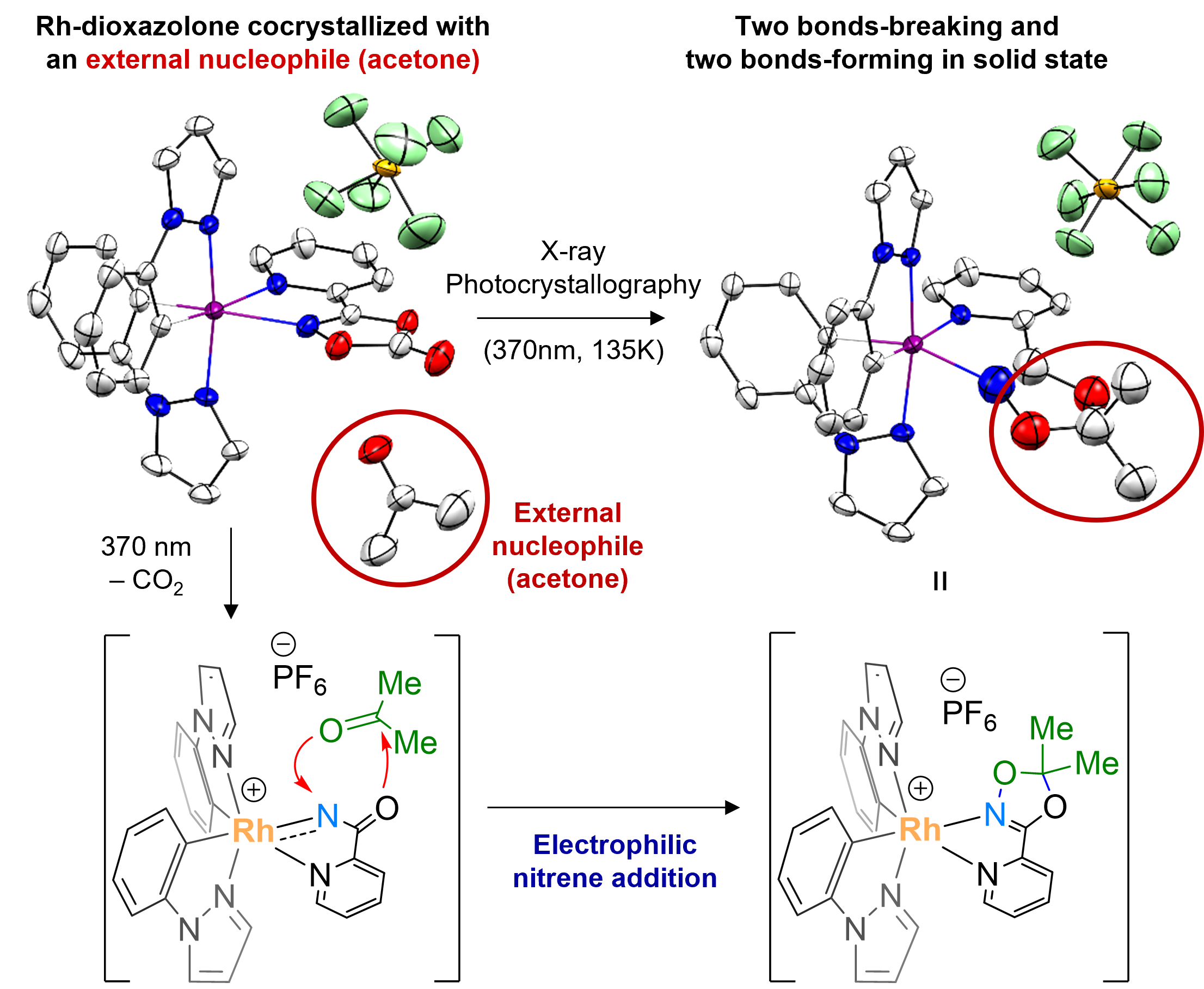
Observing the long-postulated intermediate of catalytic amination reactionsResearchers use X-ray photocrystallography to capture the key Rh-acylnitrenoid intermediate, elucidating the transition metal-nitrenoid transfer process Release Summary Text (75 words maximum)Researchers at the Institute for Basic Science (IBS) in South Korea have made a groundbreaking discovery by identifying the structure and reactivity of rhodium-acylnitrenoid intermediates in catalytic hydrocarbon amination reactions. By studying rhodium-dioxazolone coordination complex using photoinduced single crystal X-ray diffraction analysis, they captured the fleeting moment of Rh-acylnitrenoid intermediate formation. This achievement is expected to pave the way for developing highly reactive and selective catalysts for converting hydrocarbons into value-added products, with potentially wide-ranging applications in various industries. Full text of release:Led by Director CHANG Sukbok, scientists from the Center for Catalytic Hydrocarbon Functionalizations within the Institute for Basic Science (IBS) have made a breakthrough in understanding the structure and reactivity of a key intermediate in catalytic reactions. This intermediate, known as a transition metal-nitrenoid, plays a crucial role in converting hydrocarbons into amides, which are important in pharmaceuticals and materials science. In chemical reactions, intermediates are substances that are formed and consumed during the transformation of reactants into products. Hence, understanding these intermediates is crucial for improving reaction pathways and developing efficient catalysts. For example, nitrogen-containing compounds form the backbone of approximately 90% of pharmaceuticals and are essential in materials science. Therefore, identifying the intermediates involved in amination reactions, where nitrogen-based functional groups are introduced into hydrocarbon raw materials, is highly important. Researchers recognized the importance of understanding the structure and properties of reaction intermediates in amination reactions. In particular, the reactions that utilize transition metal catalysts and dioxazolone reagents were found to be highly useful for medicinal chemistry and materials science, with more than 120 research groups worldwide contributing to the development of this field. The key to understanding these reactions at the fundamental level lay in the ability to study the reaction intermediate that forms when a transition-metal catalyst binds to the dioxazolone reagent – known as metal-acylnitrenoid. These intermediate species have been notoriously difficult to study due to their highly reactive nature, which only allows them to exist for a fleeting moment. In addition, traditional catalytic reactions often occur in a solution, where the intermediary substances quickly react with other molecules, making them even more difficult to study. To tackle this challenge, the IBS team devised an experimental approach using X-ray photocrystallography. In addition, they also focused on tracking chemical reactions in solid-state rather than in liquid solutions. For this purpose they developed a new chromophoric rhodium complex with a bidentate dioxazolone ligand, where photoinduced metal-to-ligand charge transfer initiates catalytic C–H amidation of hydrocarbon sources such as benzene. Using this newly designed system, the researchers synthesized an isolable rhodium-dioxazolone coordination complex. Then, through photoinduced single crystal X-ray diffraction analysis using synchrotron radiation (Pohang Accelerator Laboratory), they managed to reveal the structure and properties of the rhodium-acylnitrenoid intermediate for the first time. Moreover, this study was designed to also achieve crystallographic monitoring of rhodium-acylnitrene transfer toward an external nucleophile all in the solid phase, which provides complete mechanistic snapshots of the nitrenoid transfer process. This groundbreaking research marks a significant step forward compared to previous research in the field of catalysis involving metal-nitrenoid intermediates. By observing metal-nitrenoid intermediates in catalytic reactions and the study provides crucial insights into their reactivity. These findings are expected to contribute to the development of more reactive and selective catalysts for hydrocarbon amination reactions in the future. Director Chang highlighted the importance of this discovery by stating, “We have experimentally captured the transition metal-nitrenoid intermediate, whose existence had only been hypothesized and was difficult to prove.” He further noted that this research would provide important clues for the design of highly reactive and selective catalysts that could be useful across various industries, possibly even contributing to the development of a ‘universal catalyst’.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20