주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
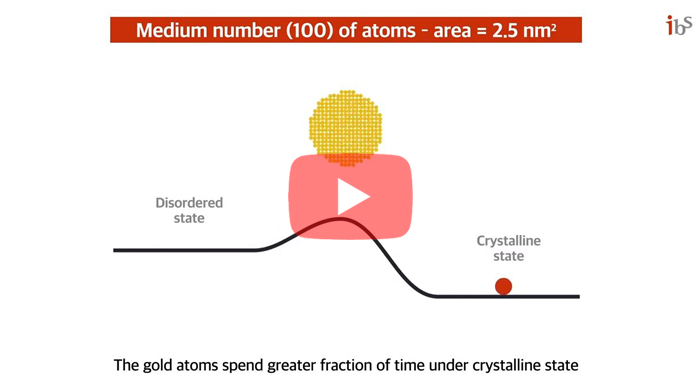
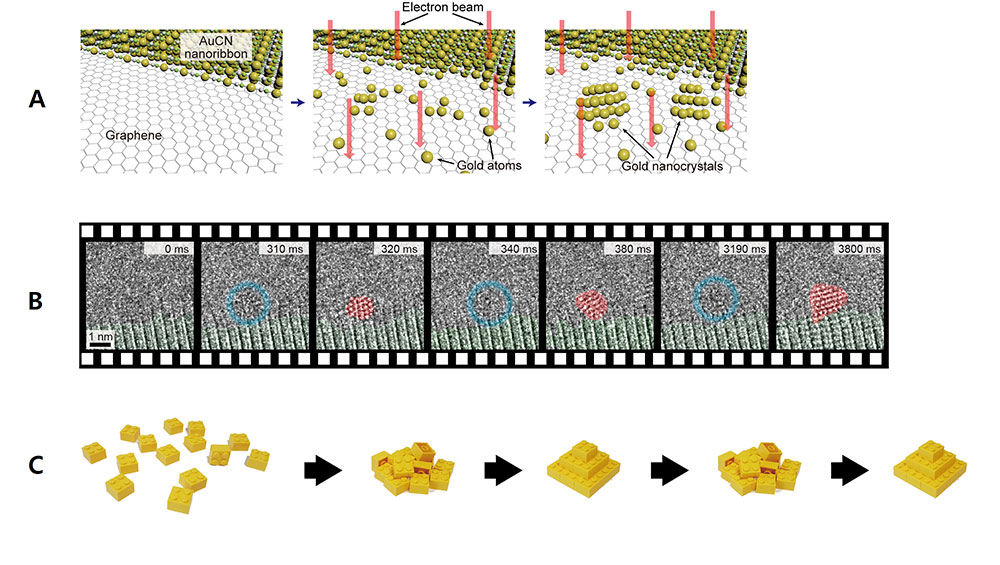
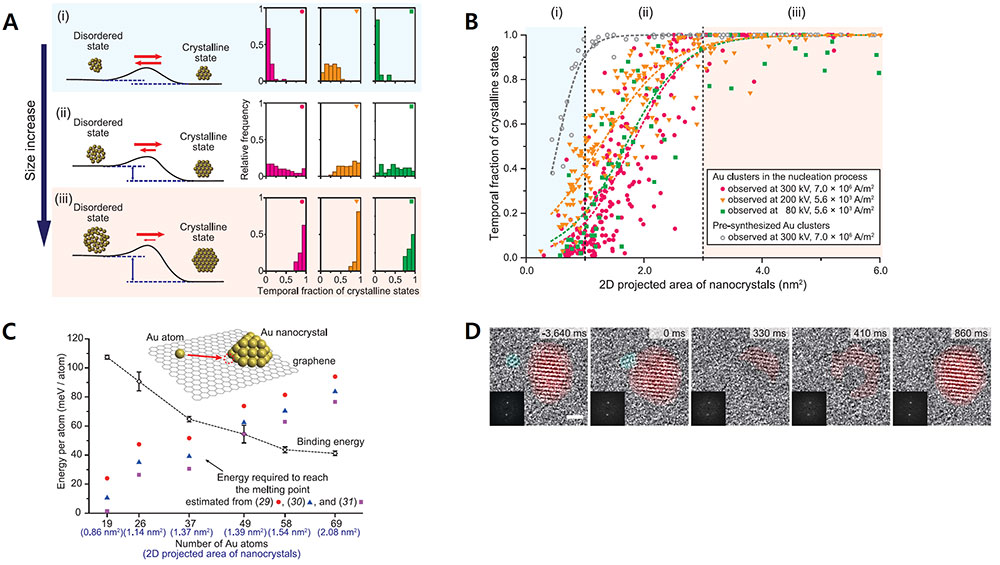
Scientists capture the moments of nascent crystal formation and growthThe crystals undergo stochastic fluctuation between crystalline and disordered states at the earliest stage of growth Conversion of most materials into organized crystalline structure starts with the nucleation process. One everyday example that many people may be familiar with is the rapid crystalization of supercooled water after the nucleation of a seed crystal. This phenomenon has been perplexing both scientists and ordinary people alike. The nucleation process, in which the atoms gather and form the smallest crystals, has been an important scientific phenomenon that has been widely studied since the late 1800s. The classical nucleation theory states that the assembly of monomers into a crystal structure occurs in a one-directional fashion. On the other hand, there have been some who suggested that a non-classical crystallization process involving metastable intermediate crystal structures may occur in some systems. However, it has been extremely difficult to confirm these theories through direct observation because the nucleation occurs very rapidly, and the size of a nucleus can be as small as a few atoms. This century-old mystery has been finally solved by an international joint research team led by LEE Won Chul, Professor of Mechanical Engineering at Hanyang University Erica Campus, JEON Sungho, Postdoctoral Researcher of Mechanical Engineering at Hanyang University Erica Campus, PARK Jungwon, Professor of School of Chemical and Biological Engineering at Seoul National University and Center for Nanoparticle Research within the Institute for Basic Science (IBS), and Peter ERCIUS from Lawrence Berkeley National Laboratory. The joint research team has succeeded in observing the moment of the initial state of nanocrystal nucleation. The scientists succeeded in filming the process where the gold atoms gather to form nanocrystals. To observe the initial state of the nucleation process, the team synthesized gold nanocrystals by emitting electron beam onto gold cyanide nanoribbons on top of a graphene membrane, which decomposes the nanoribbons into gold atoms. The synthesized specimen was observed with the high-performance transmission electron microscope (TEM) at the Lawrence Berkeley National Laboratory. The process was recorded at an atomic level spatial resolution and an ultra-high temporal resolution on a scale of milliseconds. The TEM observation showed the abrupt disappearance and reappearance of crystal lattice structures prior to the emergence of a stable crystal structure. Through careful analysis, the team ruled out some factors which may result in such observations such as the orientation, tilt, and fast rotation of nanocrystals. Therefore, the observed results appeared to indicate that the atoms making up the nucleus randomly oscillate between the disordered and crystalline states. This structural fluctuation appeared to occur spontaneously in a stochastic manner. The team’s discovery directly challenged the longstanding nucleation theory as well as a more recent nucleation theory that has been proposed in the last two decades. In addition, the team found that the stability of the crystalline state increased as the size of the nanocrystals increased. For example, the nanocrystals with 2.0 nm2 areas spent approximately half of the time existing in a crystalline state. When the crystal sizes increased to above 4.0 nm2 in area, the crystals appeared to exist most of the time under a crystalline form. In order to describe this phenomenon, the team proposed a new thermodynamic theory of crystal nucleation. The study proposed that the energy barrier between crystalline to disordered transformation tends to be very low in the earliest stage of nucleation when the cluster size is small and that it increases as more atoms are added to the structure. This can explain the spontaneous fluctuation between crystalline and disordered states in nascent crystals consisting of a few atoms. The team also pointed out in relatively smaller nanocrystals, even the addition of extra atoms can transfer enough energy into the system to transform the entire structure back to a disordered state. The energy barrier increases as the crystal grows, which reduces the probability of spontaneous reversion and stabilizes the crystalline structures in larger crystals. Regarding these findings, Prof. Jungwon Park stated that "From a scientific point of view, we discovered a new principle of crystal nucleation process, and we proved it experimentally." Prof. Won Chul Lee mentioned that "In an engineering point of view, by reproducing the initial state of the deposition process, it can be used to achieve original technology in semiconductor materials, components, and equipment." This research was published in the journal Science on January 29, 2021.
Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) - About the Molecular Foundry |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20