주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
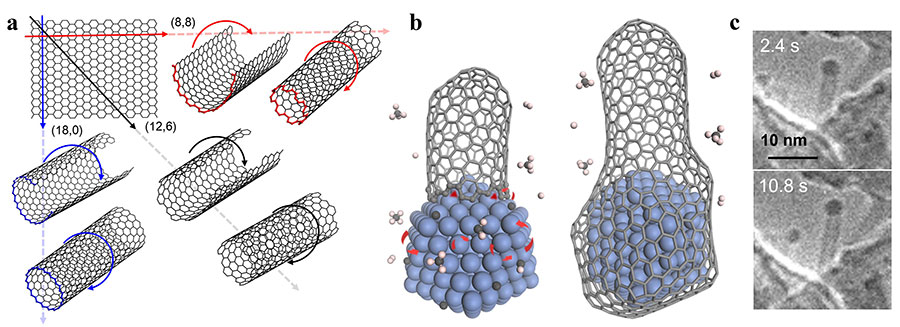
Growing carbon nanotubes with the right twist- Researchers synthetize nanotubes with a specific structure expanding previous theories on carbon nanotube growth - In a recently published paper in Science Advances, Feng Ding of the Center for Multidimensional Carbon Materials, within the Institute of Basic Science (IBS, South Korea) and colleagues, have achieved the creation of a specific type of carbon nanotubes (CNTs) with a selectivity of 90%, and expanded the current theory that explains the synthesis of these promising nano-cylinders. CNTs are incredibly strong and light nanomaterials made of carbon with superior current carrying capacity and very high thermal conductivity, making them ideal for electronic applications. Although CNTs are considered as some of the most interesting materials for the future, scientists are still struggling for their controllable synthesis. The CNTs’ shape can be compared to paper tubes: in the same way as a cylinder can be created by rolling a sheet of paper, so CNTs can be imagined as a single layer of graphite rolled up on itself. Similarly, as different tubes can be produced by rolling a paper around its long side, its short side, or diagonally at different angles. Depending on the rolling direction, a graphite layer can produce different CNT structures, some are conducting and others are semiconducting, thus selectively creating a specific type of CNT will be key for their future use, such as building energy efficient computer chips. However, CNTs are not produced by rolling, but are grown nanometer after nanometer, adding carbon at the rim of nano-cylinders, one atom at a time. Despite various studies during the last three decades, the understanding on CNT growth remains very limited and rational experimental design for the growth of specific types of CNTs is challenging. One of the most promising manufacturing methods for CNT is the chemical vapor deposition (CVD). In this process, metal nanoparticles combined with carbon-containing gases form CNTs inside a high-temperature furnace. On the tip of the tubes, the metal nanoparticles play a critical role as catalysts: they dissociate the carbon source from the gases, and assist the attachment of these carbon atoms to the CNT wall, making the tubes longer and longer. The growth of the CNT terminates once the catalyst particle is encapsulated by graphitic or amorphous carbon.
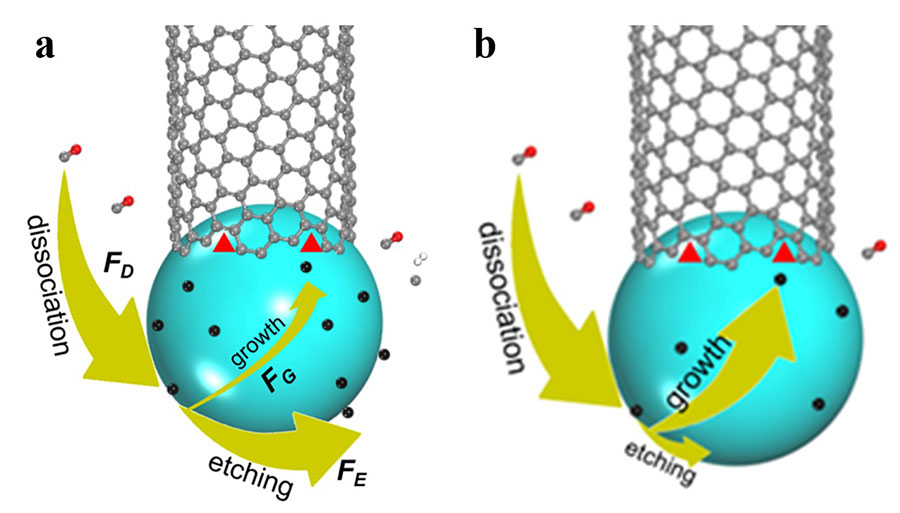
Carbon atoms are inserted onto the interface between a growing CNT and a catalyst nanoparticle, in active sites of the rim, and are available to incorporate new atoms. A previous model of CNT’s growth rate showed that the latter is proportional to the density of these active sites at the interface between CNT and the catalyst, or the specific structure of the CNT. In this study, the researchers monitored the steady growth of CNTs on a magnesium oxide (MgO) support with carbon monoxide (CO) as the carbon feedstock and cobalt nanoparticles as catalysts at 700oC. The direct experimental measurements of 16 CNTs showed how to expand the previous theory. “It was surprising that the growth rate of a carbon nanotubes only depends on the size of the catalyst particle. This implies that our previous understanding on carbon nanotubes growth was not complete,” says Maoshuai He, the first author of the paper. More specifically, carbon atoms that are deposited on the catalyst particle surface can be either incorporated on the active side of the CNT or removed by etching agents, such as H2, H2O, O2, or CO2. To explain the new experimental observations, the team included the effects of carbon insertion and removal during CNT growth and discovered that the growth rate depends on the catalyst’s surface area and tube diameter ratio. “Compared to the previous model, we added three more factors: the rate of precursor deposition, the rate of carbon removal by etching agents, and the rate of carbon insertion into a carbon nanotube wall. When feedstock dissociation cannot be balanced by carbon etching, the rate of carbon nanotube growth will no longer depend on the structure of the carbon nanotube. On the other hand, the previous theory is still valid if the etching is dominating,” explains Ding, a group leader of the Center for Multidimensional Carbon Materials.
Interestingly, the new theory of CNT growth leads to a new mechanism to selectively grow a specific type of CNTs, denoted as (2n, n) CNTs, which is characterized by the maximum number of active sites at the interface between the CNT and the catalyst. This CNT structure would correspond to rolling a sheet of graphite diagonally at an angle of around 19 degrees. “If there is no carbon etching and the carbon nanotubes growth is slow, carbon atoms on the catalyst surface will accumulate,” says Jin Zhang, co-author of the study and professor of Peking University, China. “This may lead to the formation of graphitic or amorphous carbon, which are established mechanisms of carbon nanotube growth termination. In this case, only carbon nanotubes which are able to add carbon atoms on their walls, that is with the highest number of active sites, can survive.” Guided by the new theoretical understanding, the researchers were able to design experiments that produced (2n, n) CNTs with a selectivity of up to 90%: the highest selective growth of this type of CNT was achieved in the absence of any etching agent and with a high feedstock concentration. Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20