주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
Nanoparticles “click” immune cells to make a deeper penetration into tumorsScientists proved better therapeutic results with a click reaction-assisted immune cell targeting (CRAIT) strategy Tiny nanobots flowing through the body to repair damaged cells. Once supposed to be considered as science fiction, these microrobots are becoming a reality with a slew of experimental trials. It is generally thought that nanoparticles are so tiny that they can roam freely all over the body after administration. However, this is only partly true. In a tumor, nanoparticles can make inroads into tumors only as deep as 100 µm from the vessels. The diffusion of the nanoparticles can be also hindered by several barriers, such as dense tumor tissue, high interstitial pressure, and inhomogeneous vascular distribution. Thus, cancer cells located deep in the tissue may survive, resulting in recurrence. Interestingly, it is reported that immune cells tend to accumulate at deep tumors. As tumors outgrow blood supply, immune cells are preferentially recruited to a tumor microenvironment to support the blood supply to tumors and tissue remodeling. There have been several attempts to use immune cells to deliver anti-cancer drugs to the regions inaccessible by conventional targeting approaches. Since most of them require time-consuming manipulations to extract, grow, and inject cells, this ex vivo process lowers efficacy of the treatment. Others explored ways to have antibody carrying nanoparticles target immune cells. Again, this approach proves ineffective as nanoparticles bulk up with the chemotherapy drug carried and cannot reach the designations efficiently.
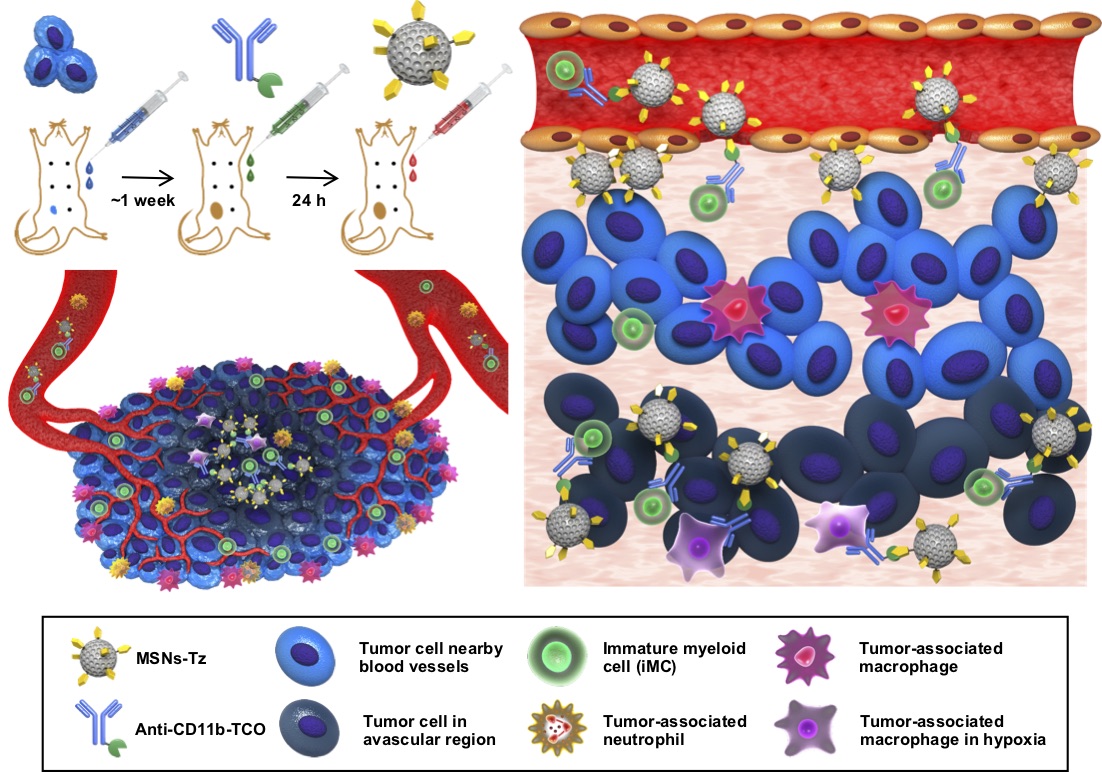
In a paper published in Journal of the American Chemical Society, the joint research team led by Director Taeghwan Hyeon at the Center for Nanoparticles within the Institute for Basic Science (IBS) in Daejeon, Dr. Seung-Hae Kwon at Korea Basic Science Institute in Seoul, and Prof. Nohyun Lee at Kookmin University in Seoul, South Korea reported a novel targeting strategy that allows deep tumor penetration of drug-loaded nanoparticles. They used a “click reaction”, a chemical reaction that easily joins molecular building blocks just as two pieces of a seat belt “click” to buckle. “Our idea was to induce the linking of immune cell-targeting antibodies to drug-loaded nanoparticles on the cells, instead of taking them up in the cells or using antibody-nanoparticle conjugates. Most other studies did so and failed to produce satisfactory results,” notes Professor Nohyun Lee, the corresponding author of the study.
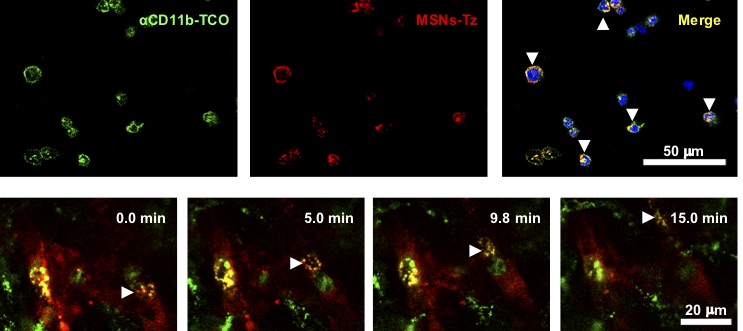
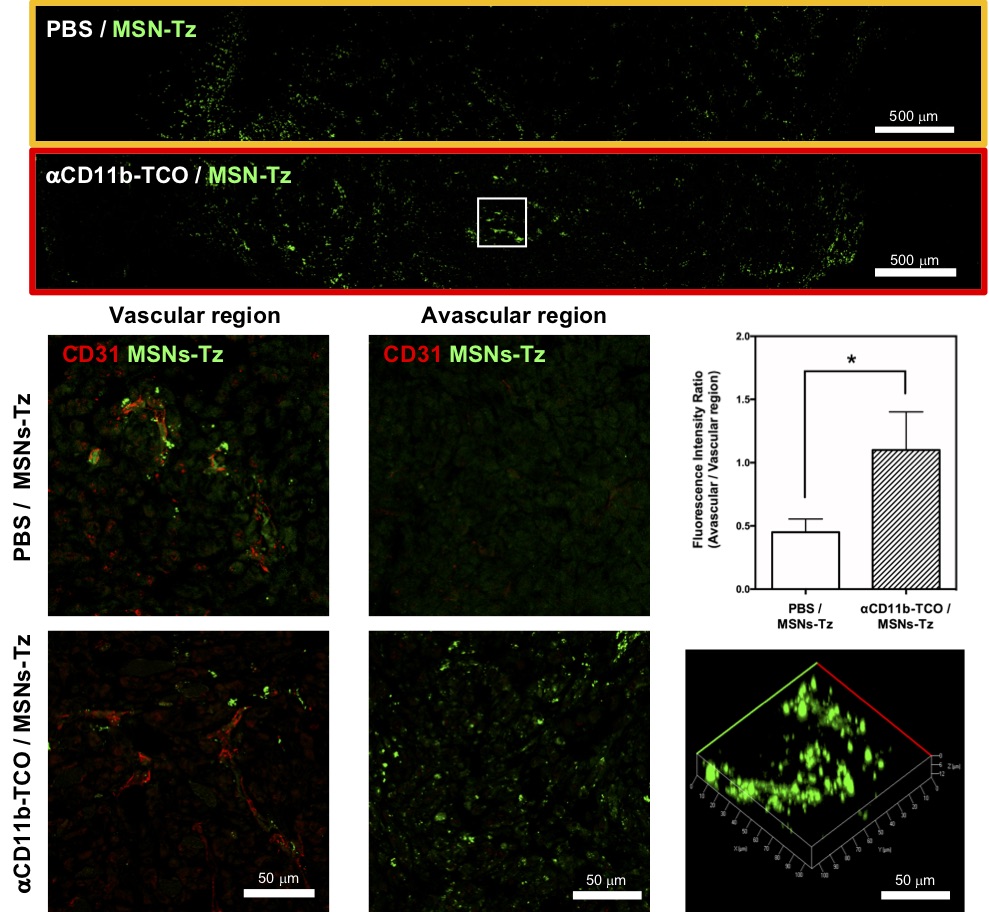
In a click reaction, chemical reagents enable an easy link of unnatural chemical groups to any site of a target protein with high site-selectivity. In the study, researchers used the click reaction between trans-cyclooctene and tetrazine. Trans-cyclooctene-functionalized antibodies are injected into mice to label tumor-infiltrating immune cells. After a certain time, tetrazine-functionalized mesoporous silica nanoparticles are administered so that they “click” to link up to immune cells. “This click reaction-assisted immune cell targeting (CRAIT) strategy successfully “invaded” the intended areas: Real-time fluorescence imaging of the tumor tissue shows that motile immune cells transport the nanoparticles as seen in Figure 2. Compared to passive targeting, the CRAIT method brought a twofold reduction in the tumor burden in aggressive breast cancer models,” explains Dr. Soo Hong Lee, the first author of the study. The nanoparticles loaded with an anticancer drug, doxorubicin, did not affect the viability and migration of the cells.
Director Taeghwan Hyeon, the corresponding author of the study says, “The intratumoral distribution of nanoparticles delivered by the CRAIT method was the key to overcoming limitations of conventional delivery methods. This study will broaden the application of nanomedicines.” Since the CRAIT method relies on the click reaction, it can be applied to various delivery vehicles including micelles, liposomes, and other nanoparticles. Additionally, if adequate antibodies are available, various circulating cells can be used as delivery vehicles. Because the circulating cells are involved in various inflammatory diseases, the coverage of the CRAIT method is not limited to cancer. The versatile CRAIT method is simple, which requires modification of antibodies and nanoparticles using well-developed bio-conjugation reaction. Dahee Carol Kim Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Nanoparticle ResearchPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20