주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
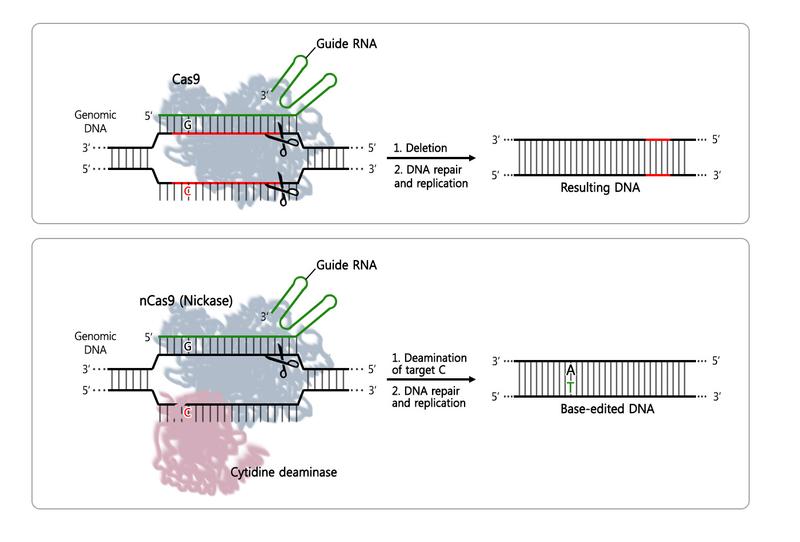
First CRISPR Single-Nucleotide Edited Transgenic Mice- IBS scientists succeeded in producing the first transgenic mice using a base editor made of cytidine deaminase fused to CRISPR-Cas9 - Cystic fibrosis, sickle cell anemia, Huntington's disease and phenylketonuria are all examples of disorders caused by the mutation of a single nucleotide, a building block of DNA. The human DNA consists of approximately 6 billion nucleotides of four types: Adenine (A), cytosine (C), guanine (G), and thymine (T). In some cases, the difference of just one nucleotide can bring serious consequences. Scientists hope to cure these diseases by substituting the incorrect nucleotide with the correct one. However, it is technically challenging to replace a single nucleotide with the current gene editing tool, CRISPR-Cas9. Scientists at the Center for Genome Engineering, within the Institute for Basic Science (IBS) have used a variation of the popular gene editing technique CRISPR-Cas9 to produce mice with a single nucleotide difference. Their findings are published in Nature Biotechnology. The most recent and highly successful CRISPR-Cas9 technique works by cutting around the faulty nucleotide in both strands of the DNA and cuts out a small part of DNA. Conversely, IBS biologists used a variation of the Cas9 protein (nickase Cas9, nCas9) fused with a protein called cytidine deaminase, a.k.a. Base Editor, which is able to substitute one nucleotide into another. In this way, no DNA deletion occurs, but just one nucleotide substitution. These types of deaminases have been developed and tested in cultured cell lines by David Liu's group at Harvard and Keiji Nishda and his colleagues at Kobe University in 2016. The IBS team advanced the technique further by applying it to mouse embryos.
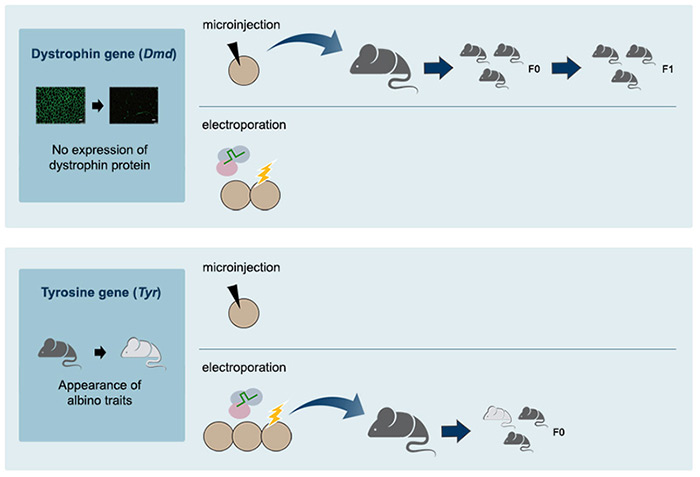
The scientists tested the CRISPR-nCas9-cytidine deaminase fusion in mice by changing a single nucleotide in the dystrophin gene (Dmd) or the tyrosinase gene (Tyr). They were successful in both cases: Embryos with the single nucleotide mutation in the Dmd gene led to mice producing no dystrophin protein in their muscles, and mice with the Tyr mutation showed albino traits. Dystrophin is indeed connected with the muscular dystrophin disease and tyrosinase controls the production of melanine.
Moreover, these single-nucleotide substitutions appeared only in the target position. This is important because it means that only the correct nucleotide is substituted. "We showed here for the first time that programmable deaminases efficiently induced base substitutions in animal embryos, producing mutant mice with disease phenotypes. This is a proof-of-principle experiment. The next goal is to correct a genetic defect in animals. Ultimately, this technique may allow gene correction in human embryos," expressed KIM Jin-Soo, Director of the Center and leading author of this study.
Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Genome EngineeringPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20