주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
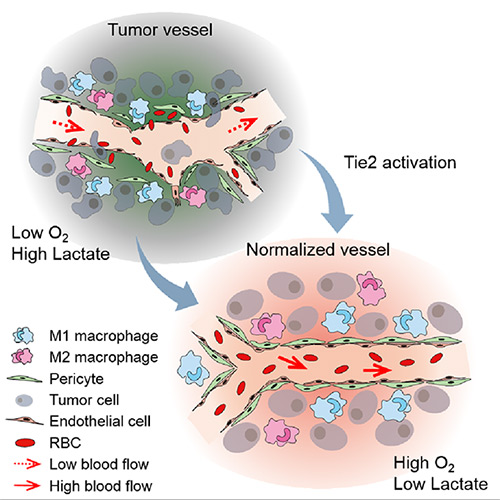
The Antibody that Normalizes Tumor Vessels- IBS scientists discover that their antisepsis antibody also reduces brain, lung and breast cancer progression in mice - An important parcel must be delivered to the correct place. It is so important that it can be a question of life or death. However, uneven streets and missing railings risk derailing it, and leaving it undelivered. This is not the trailer of a drama, but what happens to anti-cancer drugs traveling towards a tumor area, but unable to reach it because of dysfunctional blood vessels. The Center for Vascular Research, within the Institute for Basic Science (IBS) discovered the antisepsis antibody ABTAA (Ang2-Binding and Tie2-Activating Antibody) reduces tumor volume and improves the delivery of anti-cancer drugs. Published in Cancer Cell, the study demonstrates that ABTAA restores the structural and functional integrity of tumor blood vessels in three different tumor models: Breast, lungs and brain. Blood vessels inside and around an established tumor can be described as a chaotic and dysfunctional labyrinth. While the inner walls of healthy blood vessels are surrounded and supported by endothelial cells and other cells called pericytes; in an established tumor, the endothelial junctions are broken apart and pericytes also detach. Blood flowing in and out from the tumor is severely restricted and tumor vessels lacking an intact vessel wall become leaky. This microenvironment causes limited drug delivery to the tumor and leads to inadequate oxygen supply (hypoxia) and even metastasis. IBS scientists found the antibody ABTAA normalizes the tumor vessels and hence, changes the whole tumor microenvironment. "We call it normalization of tumor vessels, because it resembles closely the wall architecture of healthy, normal vessels," explains PARK Jin-Sung, first author of the study. "Tumors adapt to hypoxia and get more aggressive, so we tried to prevent this transition by normalizing tumor vessels. ABTAA changes the whole tumor environment, oxygenation status and level of lactate, so that the immune cells and drugs can reach the core regions of the tumor more easily. In this way, we created a favorable ground for tumor treatment." In an attempt to generate antibodies targeting the protein Ang2, which is specifically expressed by endothelial cells in stressful conditions like in a tumor, the team unexpectedly discovered that ABTAA has a dual function and a peculiar way of working. ABTAA not only blocks Ang2, but simultaneously activates Tie2. Tie2 is a receptor present on the cell membrane of endothelial cells. ABTAA causes Ang2 to cluster together and strongly activates Tie2 receptors. "If we activate Tie2, we can efficiently normalize tumor vessels, enhance drug delivery and change the whole microenvironment," explains KOH Gou Young, Director of the Center for Vascular Research. Several pharmaceutical companies are developing Ang2-blocking antibodies to cure cancer. However, even if these antibodies significantly inhibit tumor progression, they do not stop tumor hypoxia. Moreover, most of the anti-cancer drugs target the tumor at its early stage, when tumors are still hard to diagnose. ABTAA, instead, works with tumors that are already rooted: "When the tumor is established, hypoxia is the main driver of tumor progression. So, if we eliminate hypoxia, we make the tumor milder, by reducing its progression and metastasis," comments Koh.
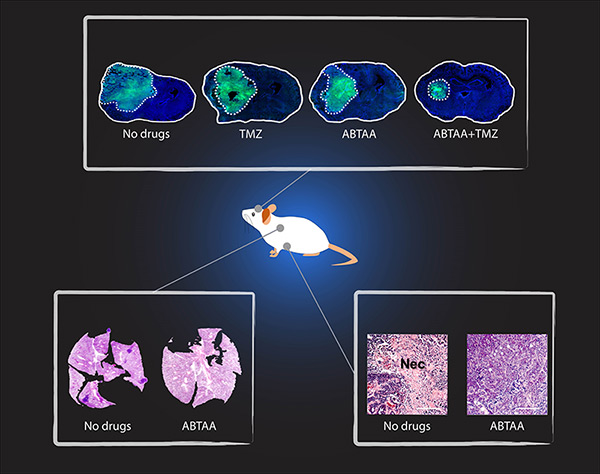
IBS researchers tested ABTAA in mice with three different types of tumors that show high levels of Ang2: Glioma (a type a brain tumor), lung carcinoma and breast cancer. They also compared the effect of ABTAA with ABA, another antibody that blocks Ang2 but misses the activating properties of Tie2. In all three cases, ABTAA was superior to ABA in inducing tumor vessel normalization, which led to a better delivery of the anti-cancer drugs into the tumor core region. Glioma is one of the so-called intractable diseases, because of its poor prognosis and treatment. IBS scientists found that the glioma volume reduced by 39% when treated with ABTAA and 17% with ABA. ABTAA profoundly reduced vascular leakage and edema formation in glioma through promoting vascular tightening. Moreover, when ABTAA was administered together with the chemotherapeutic drug temozolomide (TMZ), the tumor volume reduces further (76% by ABTAA+TMZ, 51% by ABA+TMZ, and 36% by TMZ). In the Lewis Lung Carcinoma (LLC) tumor model, the research team administered ABTAA together with a chemotherapeutic drug called cisplatin (Cpt) and observed a greater suppression of tumor growth (52%) compared with the controls and increased overall survival. Moreover, ABTAA+Cpt led to a marked increase in necrotic area within tumors. Finally, in a spontaneous breast cancer model, ABTAA delayed tumor growth and enhanced the anti-tumor effect of Cpt.
In the future, the team would like to further understand the underlying relationship between faulty blood vessels and diseases. "We would like to apply this antibody to an organ that is rich in blood vessels, like the eye; and see if this antibody can be useful to treat eye diseases such as age-related macular degeneration and diabetic retinopathy," concludes Koh.
Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Vascular ResearchPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20