주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
Highly Sensitive and Effective Tool Measures How Your Cells Grow and Divide- The improved sequencing tool from the IBS Center for RNA Research reveals dynamic changes of poly(A) tails in eggs and embryos - July 28, 2016 In the paper, published in the journal Genes and Development on July 10, the IBS research team, led by the director of the Center for RNA, V Narry KIM, presented mTAIL-seq, a hyper sensitive upgraded tool to measure poly(A) tail length of mRNAs at the genomic scale. By revealing the dynamic poly(A) regulation during the translation of RNA into protein, the study furthered our understanding as to how the fabric of life is shaped: from the color of your skin to your hair to how tall you will grow. The essential three & the building blocks of lifeAll life on Earth requires three separate but equally essential biological molecules that serve a critical function in a cell: Proteins, DNA and RNA. Proteins are the workers; they perform diverse catalytic and structural roles within cells. Both DNA and RNA carry genetic information that’s inherited from generation to generation. They are the reason why some people have black hair, others blond with blue eyes and others still with green eyes. Central dogma is a biological concept that explains how DNA and RNA interact to produce proteins. Developed in the late 50’s, by British molecular biologist Francis Crick, the concept fundamentally outlines the three stages: DNA replicates its information using many enzymes after which DNA’s encoded information is transcribed into RNA whereupon a variation of RNA is translated into proteins. During the transcribing process various forms of RNA are synthesized, each has an essential function to help create a template for future construction of protein. Messenger RNA (mRNA) is the architect and instructs transfer RNA (tRNA) and ribosomal RNA (rRNA) on how to correctly assemble amino acids that, when combined into chains, create proteins: the building blocks of all life.
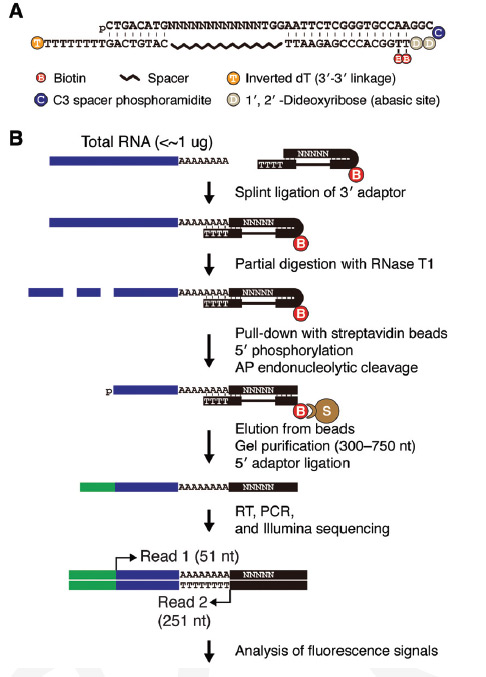
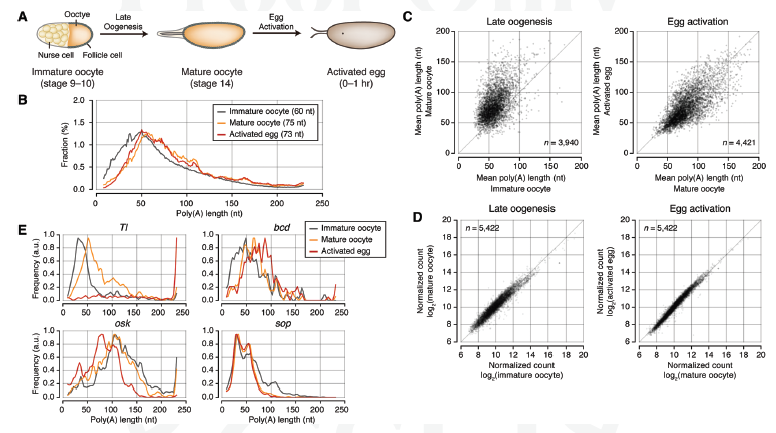
mTAIL-seq: a highly sensitive and cost effective tool to measure poly(A) tail in eggs-to-embryos developmentThe IBS team focused on oocytes (eggs) in which maternal mRNAs are deposited in a dormant state, yet to be translated into proteins. In early embryos, where transcription is silent, the activation of the dormant mRNAs is vital for the proper production of proteins; thus a deeper understanding of the regulation of maternal mRNAs can reveal how oocytes establish the maternal characteristics of embryos. The key mechanism to controlling maternal mRNAs is the regulation of poly(A) tail: A chain booster that’s added to mRNA to increase its stability and promote protein synthesis. Inside an oocyte, long poly(A) tails are added to mRNAs, allowing for them to translate and build proteins; proteins that are required for early embryonic development. The team’s upgraded tool, mTAIL-seq, allows enhanced sequencing depth for mRNA at a rate of 1,000 finer than its predecessor. First author, Dr. LIM Jaechul explains further, "We used mTAIL-seq to measure poly(A) length of maternal mRNAs in oocyte-to-embryo development. From the genomic scale analysis, we found global dynamic poly(A) tail regulation without the change of mRNA abundance." Comparing two indices for one complete pictureRibosome profiling (RPF) data measures the efficiency of translation, from mRNA to protein, the second step in central dogma. When the RNA team compared the RPF data with data acquired from mTAIL-seq they found a strong coupling between poly(A) tail length and the translational efficiency at early embryo stage. This data, according to the team’s manuscript, "suggests that regulation of poly(A) tails in oocytes shapes the maternal characteristics {translatomic landscape} of embryos, thereby directing the onset of animal development."
(A) Schematic illustration of late oogenesis and egg activation. Global poly(A) tail lengths are addressed at three different stages: immature oocyte, mature oocyte, and activated egg. (B) Global distributions of poly(A) tails at three stages. The median poly(A) tail lengths is 60 nt in immature oocytes, 75 nt in mature oocytes, and 73 nt in activated eggs. The result from biological replicates is shown in Supplemental Figure S2D. (C) Scatter plots showing the changes of poly(A) tail lengths upon late oogenesis and egg activation, respectively. The mean poly(A) tail lengths from two biological replicates were averaged. n = 2. The median of mean poly(A) tail lengths is 58 nt in immature oocytes, 76 nt in mature oocytes, and 70 nt in activated eggs. (D) Changes of mRNA abundance upon late oogenesis and egg activation measured by RNA sequencing (RNA-seq). (E) Examples of individual genes. mTAIL-seq tags were plotted in 3-nt-wide bins and then smoothened with a Hanning window (width = 5). The frequency along the Y-axis was normalized by the maximum value at each stage. "The global profiling of poly(A) tails by mTAIL-seq provides a comprehensive resource for the regulation of poly(A) tails in Drosophila {fruit flies} oocyte-to-embryo development and it help us to understand how poly(A) tail of maternal mRNA affect the production of proteins at the beginning of embryonic development," said the first author Dr. Mihye LEE. Due to the high sensitivity and low cost of mTAIL-seq not to mention its technical robustness and broad accessibility, the team think their invention will be a potent tool to improve understanding of mRNA tailing in diverse biological systems. by Neil Mannix Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for RNA ResearchPublication Repository |
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20