주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Uncovering the Secret of Insulin Growth Factor Ternary Complex | ||
|---|---|---|---|
| Embargo date | 2022-08-10 10:10 | Hits | 725 |
| Press release |
![docx 파일명 : [v4] 해외홍보자료_BCS_김효진_ver2.docx](/images/mimetype/docx.gif) [v4] 해외홍보자료_BCS_김효진_ver2.docx
[v4] 해외홍보자료_BCS_김효진_ver2.docx
|
||
| att. | |||
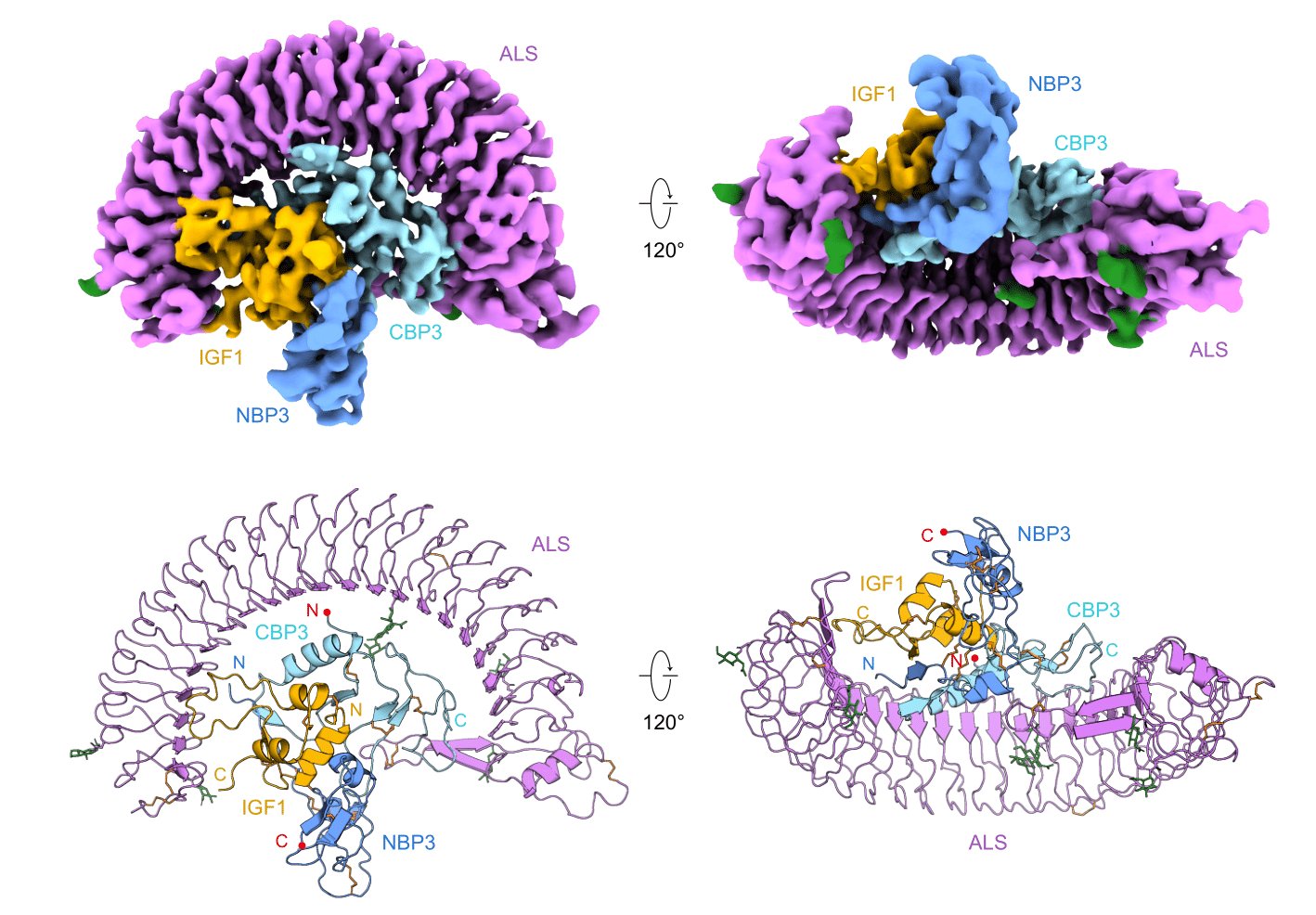
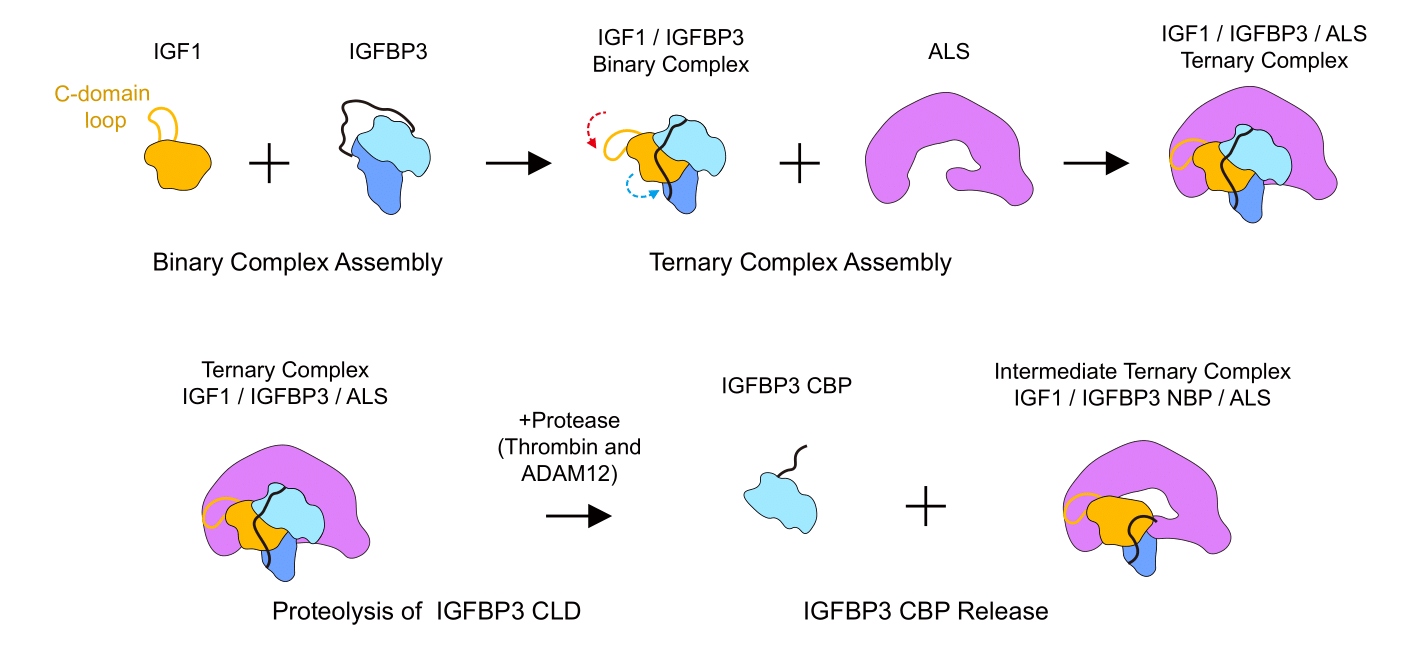
Uncovering the Secret of Insulin Growth Factor Ternary Complex- Scientists determined the cryo-EM structure of IGF Ternary complex and its assembly & activation mechanism - Insulin-like growth factor (IGF) is a hormone that greatly influences growth in fetuses and children, but also body maintenance and metabolism in adults. IGF regulates cell proliferation, differentiation, and survival by activating IGF receptors distributed in cell membranes of various tissues. However, IGF is so unstable that its half-life is less than 10 minutes in its free state. For, this reason more than 70% of IGF circulating in the body is bound to IGFBP (IGF Binding Protein) and ALS (Acid Labile Subunit). This IGF/IGFBP/ALS ternary complex is stable for a much longer duration of 12 hours and therefore serves as a carrier for IGF. In addition, the ternary complex also plays a role in regulating IGF so that it can act only when needed. However, the structure of the IGF/IGFBP/ALS ternary complex has not been well studied, making it difficult to understand how the complex is assembled and how IGF is activated. Now, a team led by Professor KIM Ho Min at the Center for Biomolecular and Cellular Structure within the Institute for Basic Science (IBS) in Daejeon, South Korea, determined the structure of IGF1/IGFBP3/ALS ternary complex for the first time. It is believed that this structural information could broaden the understanding of growth-related diseases such as growth hormone deficiency and ALS deficiency. The stable ternary complex was successfully expressed and purified through an animal cell expression system and a series of purification processes. The cryogenic transmission electron microscope (cryo-EM), data hub, and supercomputing resources of IBS were used to obtain its high-resolution three-dimensional molecular structure. It was discovered that IGF1 was surrounded by IGFBP3 to first form a binary complex. Then, ALS once more wraps this binary complex like a parachute, which protects IGF1 from being easily degraded within the body.
Armed with the new knowledge of the protein structure, the researchers were now able to understand the structural basis for many diseases associated with the mutations in these proteins. In particular, many known point mutations in ALS were found to cause disease by inducing misfolding of ALS and hindering the formation of the ternary complex. On the other hand, known point mutations in IGF did not appear to have any negative effects on their ability to form complexes, but rather cause disease by decreasing IGF’s affinity to IGF receptors. The researchers did not stop there, as they further explored how this ternary complex is assembled. First, the researchers ran SDS-PAGE of ALS, IGFBP3, and IGF1 proteins to verify if the complex is or isn’t formed. They also fused GFP (green fluorescent protein) onto the IGF1 protein and used fluorescence detection chromatography to trace the GFP labeled protein as they succeed or fail to form complexes with the other two proteins. From this, they learned that IGFBP3 or IGF1 alone cannot form a binary complex with ALS. Moreover, it was discovered that IGF must first form a binary complex with IGFBP, and only after that, both proteins can bind to ALS to properly assemble a ternary complex. Furthermore, the researchers modified IGFBP to better determine the role of specific domains of the protein. When the N terminal domain (NBP) was removed, the protein was shown to be incapable of binding to IGF, hinting that the domain is required for the initial binary complex formation. The researchers also substituted the central-linker domain (CLD3) of the IGFBP with different variants. Contrary to NBP, all variants of CLD domains allowed the formation of the binary complex with IGF1. However, it was shown that some CLD variants allowed the modified IGFBP to bind to ALS even without IGF. This means that it is likely that the formation of the initial IGF1/IGFBP3 binary complex allows for some conformational change to the CLD3 domain to facilitate ternary complex formation with ALS. In addition, the researchers monitored how the complex can be disassembled to make IGF bioavailable. In order to do so, they treated both IGF/IGFBP binary complex and the final ternary complex using a protease called thrombin. After the proteolysis, it was observed that the C-terminus domain (CBP) of IGFBP is released, and an unstable intermediate ternary complex is formed. This process was believed to be an important step in activating the IGF, which is otherwise inactive when it is bound within a complex. The first author KIM Hyojin states, “The first and foremost step before investigating further processes is to understand the structure. This structure-based approach and proper biochemical assays allowed us to unveil the mechanism of IGF complex formation and activation.” “This new knowledge of molecular structure and activation mechanism of the IGF ternary complex is expected to greatly contribute to the development of therapeutic agents and research related to adolescent growth or IGF-related diseases,” explains Professor KIM Ho Min, the corresponding author of the study.
This work was published in Nature Communications on July 30th. Notes for editors
- Reference
- Media Contact
- About the Institute for Basic Science (IBS)
|
|||
|
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20