주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| 제목 | Fluorine Speeds Up Two-Dimensional Materials Growth | ||
|---|---|---|---|
| 보도일 | 2019-07-16 00:00 | 조회 | 1498 |
| 연구단명 |
Center for Multidimensional Carbon Materials |
||
| 보도자료 | |||
| 첨부 | |||
Fluorine Speeds Up Two-Dimensional Materials GrowthBy spatially confining fluorine, scientists could activate feeding gases Back in 2004, the physics community was just beginning to recognize the existence of truly two-dimensional (2D) material, graphene. Fast forward to 2019, scientists explore a breadth of different 2D materials, expecting to uncover more of their fundamental properties. The frenzy behind these new 2D materials lies in their fascinating properties: materials thinned down to only a few atoms work very differently from their 3D version. Electrons packed into the thinnest-ever layer show distinctive characteristics apart from being in a “loose net”. Also being flexible, 2D materials could feature distinctive electrical properties, opening up new applications for next-generation technologies, such as bendable and wearable devices. Then, what is the catch? Many parameters such as temperature, pressure, precursor type, and flow rate need to be factored into the CVD synthesis of 2D materials. With multiple reactions involved, it is extremely difficult to optimize all these factors during the reactions and find their best combinations. That being said, 2D material synthesis is hardly controllable. Scientists have tried to accelerate the growth of 2D materials by adopting different substrates, feedstocks, or temperature. Still, only a few types of 2D materials can be synthesized into large area high quality films. Scientists from the Center for Multidimensional Carbon Materials (CMCM), within the Institute for Basic Science (IBS) at the Ulsan National Institute of Science and Technology (UNIST), in cooperation with researchers at Peking University (PKU), and University of Electronic Science and Technology of China (UESTC), demonstrated that fluorine, having the strongest tendency to attract electrons (i.e. electronegativity) in all elements, can speed up the chemical reaction to grow three representative 2D materials; graphene, h-BN, and WS2. Fluorine requires only one electron to attain a high stability. Also, having seven electrons in the outermost orbit of an atom, the distance at which these valence electrons reside is the minimum compared with other elements. This means the valence electrons of fluorine are bound to the atom more strongly than any other atom making fluorine the most active element in the periodic table.
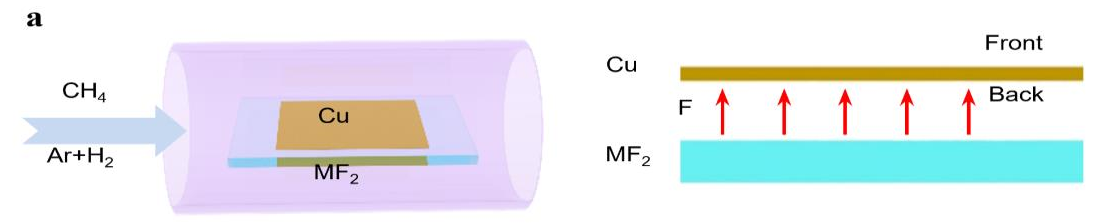
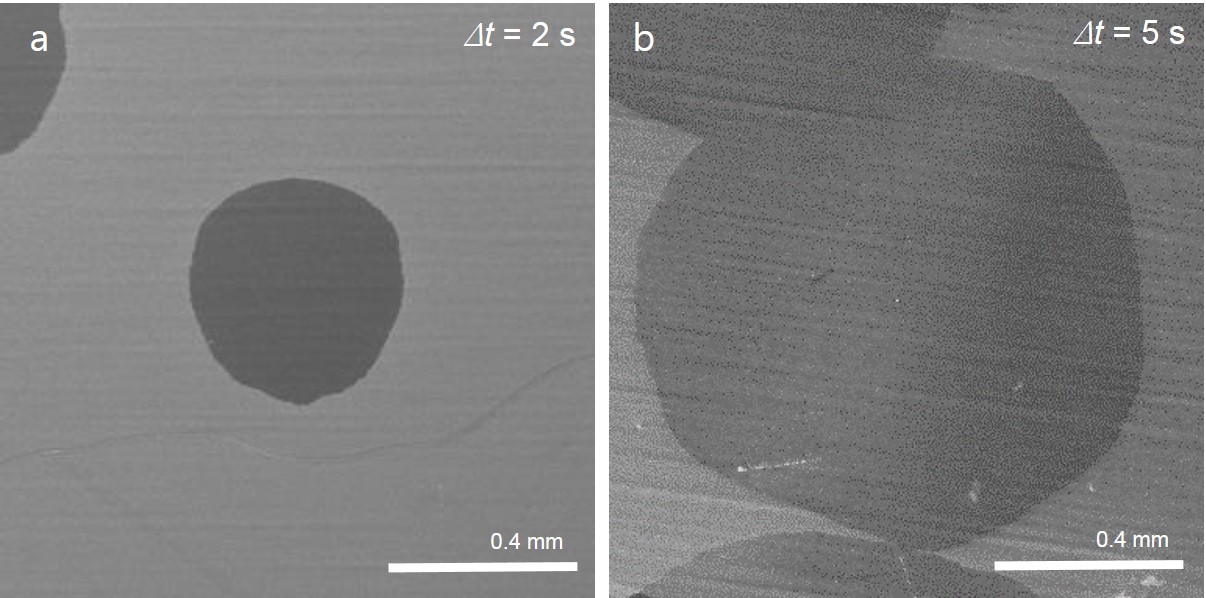
In fact, active gases such as hydrogen or oxygen are broadly used to tune the growth of graphene and other 2D materials. “Why not then the most active element, fluorine? The highest electronegativity allows fluorine to form bonds with nearly all the atoms in the periodic table, so it is expected to change the reaction routes of many chemical processes,” said Professor Feng Ding, the corresponding author of this study. Experimentally, it is not preferable to introduce fluorine during a material’s growth as fluorine gets highly toxic in the reactor. To resolve the problem, instead of using fluorine gas directly, the scientists spatially confined the fluorine supply so that only the minimum amount of fluorine is consumed. They placed a metal fluoride substrate (MF2) below a Cu foil with a very narrow gap in between. At a high temperature, fluorine radicals are released from the fluoride surface and spatially trapped in the narrow gap between the Cu foil and the metal fluoride substrate. Surprisingly, such a simple change leads to a record growth rate of graphene at 12 mm per minute. To put this rate in perspective, this new approach reduces the time of growing a 10 cm2 graphene from 10 minutes with previous methods, now down to only 3 minutes.
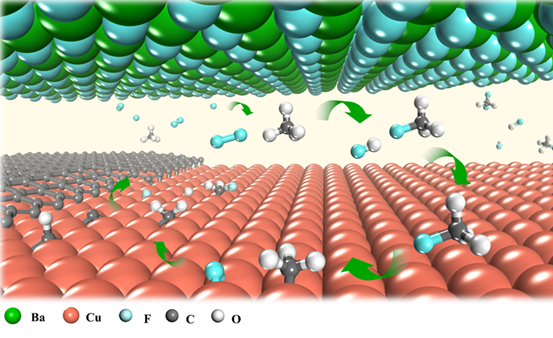
The introduction of local fluorine entirely changes the methane decomposition route. As the fluorine released from the metal fluoride surface easily reacts with methane gas, there will be a sufficient amount of CH3F or CH2F2 molecules in the gap between Cu and BaF2 substrates. These molecules could decompose on a Cu surface much more easily than CH4 does. In other words, they feed the graphene growth better by supplying more active carbon radicals (i.e. CH3, CH2, CH and C). Further experimental studies showed that the local fluorine supply strategy could greatly accelerate the growth of other 2D materials, such as h-BN and WS2, as well. The scientists investigated how spatially confined fluorine is able to accelerate the growth of 2D materials. Theoretical studies revealed that fluorine, being highly reactive readily interact with methane molecules. The existence of fluorine leads to the formation of CH3F or CH2F2 molecules. These highly active molecules can then be more easily decomposed on the Cu foil surface, which greatly accelerates the carbon supply for fast graphene growth.
Although the detailed mechanism of fluorine boosting the growth of h-BN and WS2 is not clear, the authors are confident that the presence of fluorine could significantly modify the reactions of 2D materials’ growth. “We envision that this local fluorine supply will readily facilitate fast growth of broad 2D materials or enable the growth of new 2D materials which is very difficult to be realized by other methods.” noted Professor Feng Ding. In addition to the fluoride, there are abundant kinds of substrates like sulphides, selenides, chlorides or bromides that might be used as local supply sources of different active materials, which provides wide enough platform to modulate the growth of broad 2D materials. Dahee Carol Kim Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
|||
| 다음 | |
|---|---|
| 이전 |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20