주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Increasing Lifespans by Changing Our RNA | ||
|---|---|---|---|
| Embargo date | 2015-07-21 04:00 | Hits | 7856 |
| Press release | |||
| att. | |||
|
Increasing Lifespans by Changing Our RNA Altering RNA helicases in roundworms doubles their lifespan, a similar technique could be used on human cells
July 20, 2015
The things we do to extend our lives--quitting smoking, cutting back on carbs, taking up jogging --all have some impact on our longevity, if only just a little. But no matter how hard we work towards chasing the dream of forever staying fit and youthful, our efforts all end the same way and we must come to terms with the fact that we are mortal beings living on a finite timeline. There is nothing we can do to stop the aging process, and most things people do only serve to delay the inevitable: we can’t stop death.
If someone was going to attempt to stop it, what would be the first step? Researchers at the Center for Plant Aging Research with support from the Institute for Basic Science (IBS) in Korea have made a breakthrough in decoding the aging process and how to dramatically slow it down.
Our bodies are programmed to grow rapidly when we are young, mature into adults, and then at a certain age the regeneration and repair of our cells, tissue and organs grinds to a halt. All the mechanisms are not yet completely mapped out, but the IBS team has made several significant steps toward understanding how the lifespan of a cell is regulated.
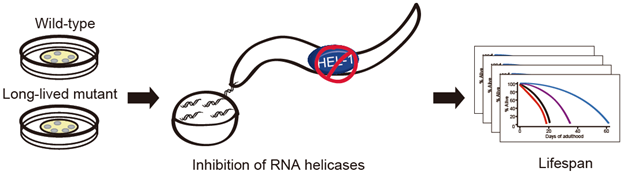
The team tested the cells of a specific roundworm, Caenorhabditis elegans, which despite being only 1 mm in length, share some of the same cellular attributes as humans. While looking into the roundworm cells, they focused their attention on RNA helicases, a family of enzymes that regulate the function of RNA. The helicases are well understood but their function in relation to the aging process has not yet been fully explored. IBS Center for Plant Aging Research Director Hong Gil Nam looked specifically at a helicase called HEL-1 and discovered that its inhibition has the property of promoting longevity in roundworms.
To identify which helicases they needed to focus their attention on, the team targeted each of the 78 RNA helicases to see what the effects would be. They noticed that the result of altering more than 30 RNA helicase genes actually significantly decreased life expectancy. They realized that they wouldn’t be able to alter every single one of the 78 RNA helicases in order to increase lifespan. Each RNA helicase played a different and important role and needed to be switched on or off individually.
Figure 1. The inhibition of RNA helicases leads to a longer lifespan in the roundworm Caenorhabditis elegans
The team used a mutated form of the roundworm in which they restricted a gene called daf-2 which is responsible for the rate of aging, reproductive development, resistance to oxidative stress, thermotolerance, resistance to hypoxia, and resistance to bacterial pathogens. In this case the daf-2 gene was altered so its IIS (insulin/insulin-like growth factor 1 (IGF1) signaling) would be restricted. These daf-2 mutants display increased resistance against diverse stresses, including heat stress, pathogenic bacteria, and oxidative stress and most importantly, the daf-2 mutants displayed double the lifespan compared to wild Caenorhabditis elegans roundworms.
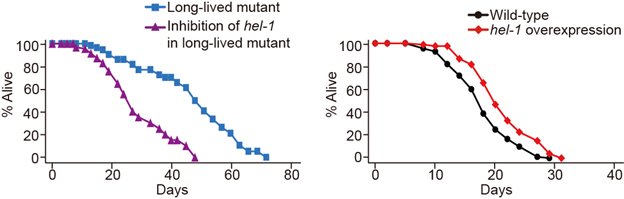
Figure 2. Left: Lengthened lifespan of mutant vs. HEL-1 inhibited mutant. Right: Wild C. elegans vs. HEL-1 overexpression.
The team believes that HEL-1 may act as a transcription regulator, which control how cells convert DNA to RNA since other RNA helicases do the same thing now. According to the team, “In contrast to the expectation that RNA helicases have general housekeeping roles in RNA metabolism, our findings reveal that the RNA helicase HEL-1 has specific roles in a specific longevity pathway.”
Even if immortality isn’t an immediate result of this work, there are other possible applications. Something called DDX39 (the mammalian version of the roundworm’s HEL-1) is found in increased levels in the frontal cortex of patients with Alzheimer’s disease. The ability to regulate DDX39 and other RNA helicases may give us an insight into finding the ability to control Alzheimer’s disease, among other brain disorders.
Using the technique of altering RNA helicases to extend life in humans looks promising as human and roundworm both have HEL-1 and IIS which can be manipulated in similar ways. It isn’t clear if the same mechanism is responsible for cellular aging regulation in humans, but evidence suggests that it might be. This research hasn’t given humanity a cure to any diseases or made any claims of human life extension but it is an important first step in more fully understanding the lifecycle and function of cells.
By Daniel Kopperud
Notes for editors - References Mihwa Seo, Keunhee Seo, Wooseon Hwang, Hee Jung Koo, Jeong-Hoon Hahm, Jae-Seong Yang, Seong Kyu Han, Daehee Hwang, Sanguk Kim, Sung Key Jang, Yoontae Lee, Hong Gil Nam, and Seung-Jae V. Lee (2015), RNA helicase HEL-1 promotes longevity by specifically activating DAF-16/FOXO signaling in Caenorhabditis elegans, Proceedings of the National Academy of Sciences, DOI:10.1073/pnas.1505451112
- Media Contact For further information or to request media assistance, please contact: Mr. Shi Bo Shim, Head of Department of Communications, Institute for Basic Science (+82-42-878-8189; sibo@ibs.re.kr) or Ms. Sunny Kim, Department of Communications, Institute for Basic Science (+82-42-878-8135; Sunnykim@ibs.re.kr)
- About the
Institute for Basic Science (IBS)
|
|||
|
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20