주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Scientists’ Talk on DNA Repair | ||||
|---|---|---|---|---|---|
| Name | Department of Communications | Registration Date | 2015-11-20 | Hits | 3039 |
| att. |
 thumb.jpg
thumb.jpg
|
||||
|
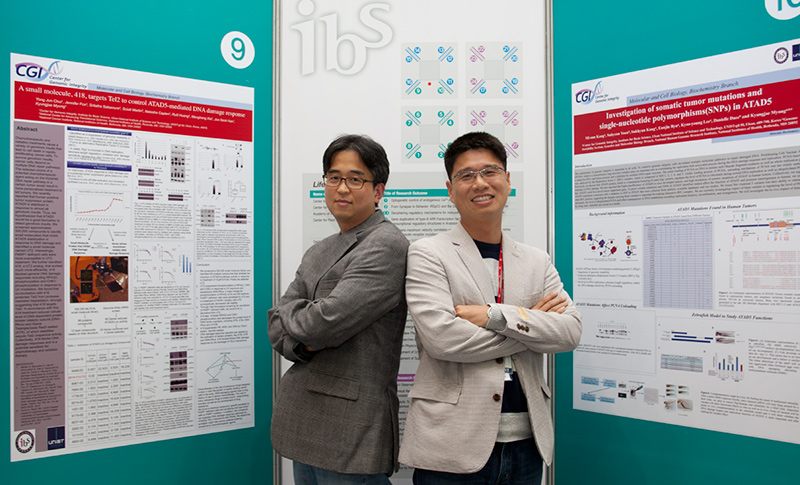
Scientists’ Talk on DNA Repair Research Fellows KIM Tae-Moon and LEE Kyoo-Young, from the IBS Research Center for Genomic Integrity, talk to IBS People about DNA structure and repair
How do cells repair damaged DNA? Three scientists, Tomas Lindahl at Francis Crick Institute and Clare Hall Laboratory in the UK, Paul Modrich at Duke University and Aziz Sancar at University of North Carolina in the US, answered this question, won the Nobel Prize in Chemistry 2015. UTHSCSA, NIH and finally IBS“The three winners of 2015 Nobel Prize in Chemistry biochemically identified DNA damage and repair mechanisms by using purified proteins. With the very classical approaches, they demonstrated how cells repair damaged DNA . It is indeed pioneering research,” said Dr. KIM Tae-Moon.
DNA, although considered extremely stable in the past, is inherently unstable. Cells keeps their genomes stable by constantly repairing DNA damage against internal or external threats. Dr. KIM is an expert on DNA repair and had worked at the University of Texas Health Science Center at San Antonio (UTHSCSA) before joining IBS. At the UTHSCSA, he studied RAD51 and BRCA2, two genes involved in the pathways to repair the double-strand break (DSB), then most deleterious type of DNA damage. “Using genetically modified mouse embryonic stem cells, my colleagues and I demonstrated the critical role of RAD51 and BRCA2 at the replication fork as well as in repairing the DSB,” said Dr. KIM. “RAD51 is associated with DNA repair, contributing to genomic stability. But, ironically we observed that it is also involved in genome instability,” he explained. The finding was published in Nature in 2013. For Dr. KIM, IBS has several merits over the UTHSCSA when it comes to research: “By observing phenotypes of genetically modified mouse ES cells and animal models, phenotypic studies could be nicely done at the UTHSCSA, but missing the mechanic studies. But at IBS, mechanismic investigations associated with cellular phenotypes could be easily performed at the molecular level thanks to well-established research infrastructure.” Dr. LEE Kyoo-Young worked at the National Health Institutes (NIH) in the US for Director MYUNG as a post-doctoral researcher. His research topic focused on PCNA and ATAD 5, two important genes associated with DNA replication and repair. DNA, according to Dr. LEE, is like a railroad on which PCNA, the train, runs. This doughnut-shaped train gives a ride to DNA polymerases, enzymes that synthesize DNA, as a cargo and moves them fast. “Without PCNA, DNA polymerases cannot synthesize DNA long enough. 100 or more proteins associated with DNA replication and repair are known to interact with PCNA,” he explained. His research has mainly focused on various aspects of PCNA. For example, he investigated how the ring of PCNA open and close to encircle DNA and how the activity of PCNA is regulated when ubiquitin, a small protein that changes other protein’s function or stability, is attached to PCNA. DNA Repair: relationship with aging and cancer preventionDr. LEE had also worked at the R&D Center of LG Life Sciences developing new pharmaceutical drugs. His main job was identifying new drug candidates in the early stage of drug discovery, and verifying the usefulness of those compounds through animal testing in order to develop treatments for metabolic disorders such as diabetes and obesity. ATAD5, the other gene of Dr. LEE’s interest at IBS, is associated with cancer. “ATAD5 prevents tumor development. ATAD5 mice models developed various cancers when their ATAD5 was mutated,” he explained. “Mutations of this gene are also observed in endometrial or colorectal cancer patients. We are investigating molecular mechanisms of ATAT5 associated with tumor prevention,” he added. Currently, the Center for Genomic Integrity is studying whether genes such as RAD51 and ATAD5 prevent tumor development, and if so, how they function as tumor suppressor. The center is now developing genetically modified knock-in mice models for ATAD5 and RAD51 respectively and will use them to tackle the questions mentioned above. In addition to cancer research, the center is also interested in looking at a possible link of DNA replication and repair to aging processes. DNA replication creates constant stress in cells. Genomes must be replicated without harmful mutations for a successful cell division, but replication-related stress naturally occurs during the process, eventually leading to DNA damage. If this damage is not repaired properly and a certain repair pathway becomes defective, genomes gradully become unstabley. Mutations and destabilized genomes, caused by increasing replication stress, may induce cellular aging, and as a result, real aging processes in living organism.
“There is a hypothesis suggesting a possible link replication stress and aging,” said Dr. KIM. “Genes that play a critical role at DNA replication forks as well as in DNA repair were found mutated in premature aging syndrome, so we are expecting to observe phenotypes related to aging in genetically modified mouse models for DNA repair genes” he added. Mutations of RAD51 were also found in humans with exceptional lifespans. Using a high throughput chemical screening method, the center has identified small molecules that selectively kill cancer cells in patients who have a mismatch repair defect and now is exploring the potential clinical applications of the molecules. A high throughput chemical screening simultaneously tests thousands to hundreds of thousands of compounds to find candidate chemical to kill DNA-defective cancer cells. The center measures the activity of ATPase to indirectly gauge its impact on cell viability. Good News to People like Angelina Jolie, who are at higher risk of breast cancerAfter testing positive for BRCA1 mutations, the famous Hollywood actress Angelina Jolie had her breasts and ovaries removed as a preventative measure. Her actions drew great attention and sparked controversy. There were no other options, she argued, because BRCA mutations are closely associated with a far higher risk of developing breast and ovary cancer. Recently, PARP1 inhibitors, treatments for breast cancer patients who have faulty BRCA genes have been approved by the Food and Drug Administration (FDA) in the US. “In five to ten years, a deeper understanding of genetic interactions between DNA repair pathways and cancer-related pathways may enable the development of various anti-cancer drugs” said Dr. KIM. Will there be another Nobel Prize honoring DNA repair research? 2015 Nobel Prize in Chemistry went to scientists who made fundamental discoveries on DNA repair. Considering the recent preference of the Nobel Committees for medical research, anyone who proves with animal models that DNA repair pathways can be exploited to treat certain diseases like cancer and evenually succeeds in its clinical applications may receive another Nobel Prize in the field of DNA repair, according to Dr. KIM. The Center for Genomic Integrity is studying how DNA repair pathways maintain genome stability to prevent cancer and aging, and hoping to translate its discoveries into the clinics. It may be premature to talk about a Nobel Prize, but the research at IBS can contribute to cancer prevention and treatment in the future. |
|||||
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20