주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
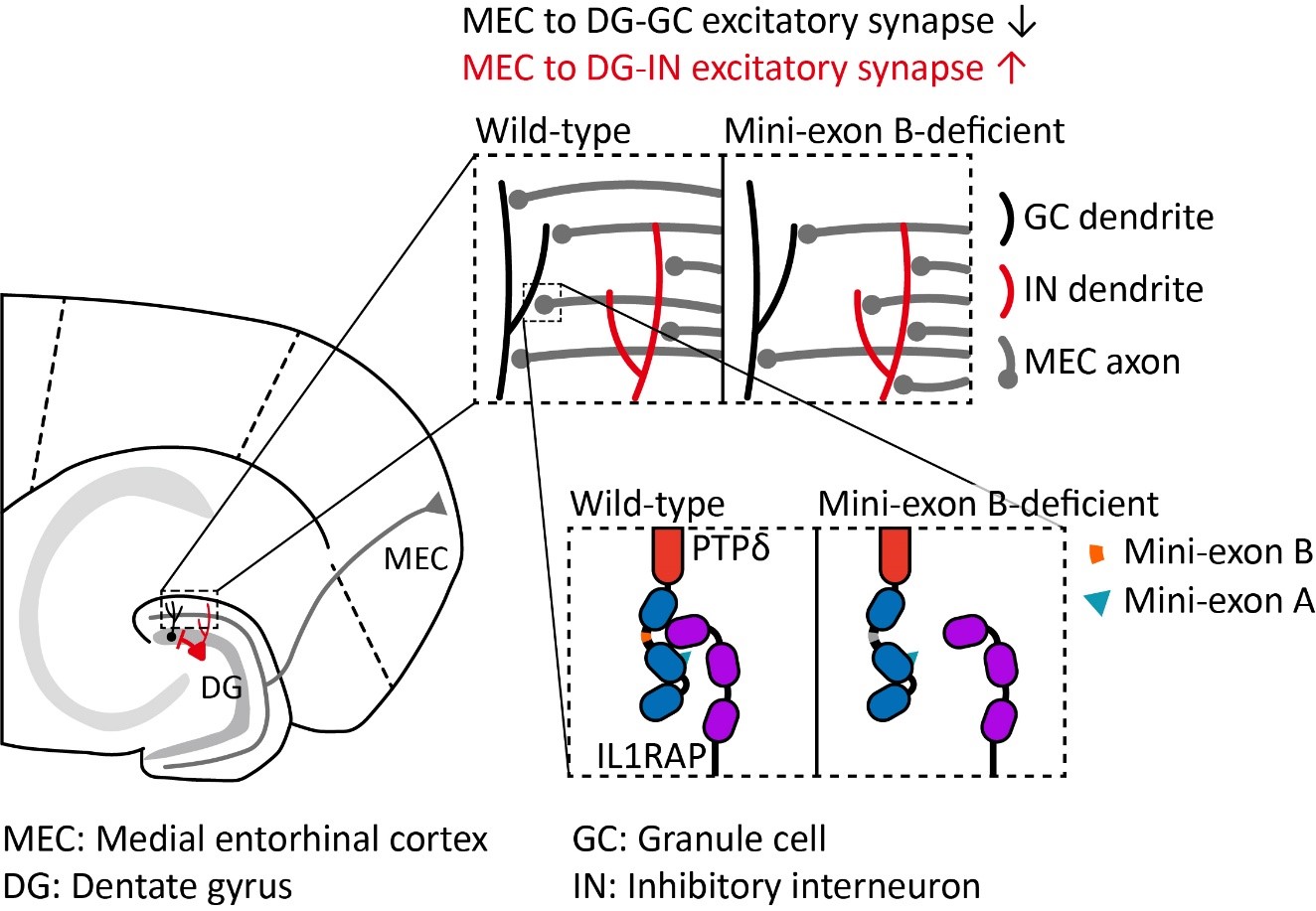
Tiny Genetic Switch Found to Control Brain Balance and Behavior– IBS researchers uncover how a four-amino-acid "mini-exon" shapes brain circuits - and may hold clues to mental health disorders – Researchers at the Institute for Basic Science (IBS) have identified a remarkably small but critical piece of genetic code that helps determine how brain cells connect, communicate, and function. The discovery not only deepens our understanding of how the brain’s wiring is built but may also explain the origins of several neurological and psychiatric conditions. The study, conducted by the Center for Synaptic Brain Dysfunctions at IBS and led by Director KIM Eunjoon (Distinguished Professor at KAIST), focuses on a protein called PTPδ—a key molecule that helps neurons form synapses, the connections that allow brain cells to pass signals. While PTPδ has already been linked to disorders such as autism spectrum disorder (ASD), ADHD, OCD, and restless leg syndrome, the researchers have now zoomed in on a previously unstudied detail: a tiny segment known as mini-exon B. This mini-exon is created through a process called alternative splicing, in which cells include or exclude specific snippets of genetic material to slightly alter the structure—and function—of a protein. Mini-exon B is just four amino acids long, yet the team found it plays a surprisingly powerful role in brain development and behavior. A Closer Look at the Brain’s Synaptic “Glue” he brain’s ability to think, feel, and move depends on a delicate balance of electrical and chemical signals. These signals travel across synapses, where one neuron passes a message to the next. Proteins like PTPδ help these synapses form properly by acting like molecular Velcro—linking neurons together with precise alignment. In their study, the researchers genetically engineered mice to delete mini-exon B from the PTPδ gene. The results were dramatic: Mice missing mini-exon B entirely had a survival rate of less than 30% after birth, highlighting its essential role in early brain development and viability. On the other hand, mice with one copy of the gene altered survived into adulthood but displayed clear behavioral changes, including anxiety-like behavior and reduced movement. The brain recordings in these mice also showed a misbalance in synaptic activity. Granule cells—neurons responsible for processing information—received weaker excitatory input, while interneurons, which help keep brain activity in check, received stronger excitatory signals. This excitation-inhibition imbalance is a hallmark feature of various neurodevelopmental and psychiatric disorders. Molecular Clues: A Lock-and-Key Partnership To uncover how this tiny segment affects brain signaling, the researchers examined the proteins interacting with PTPδ. They discovered that PTPδ forms a molecular complex with another protein called IL1RAP—but only when mini-exon B is present. Without this mini-exon, PTPδ loses its ability to engage IL1RAP, disrupting a critical pathway for forming excitatory synapses. This interaction turned out to be cell-type specific, meaning it behaves differently depending on which neurons are involved. This level of specificity explains why the deletion of mini-exon B affects some parts of the brain more than others. Director KIM Eunjoon remarked, “This study illustrates how even the tiniest genetic element can tip the balance of neural circuits. It’s a compelling reminder that errors in alternative splicing could have profound consequences in brain disorders.” Implications for Human Brain Disorders This is the first in vivo study to demonstrate the function of PTPδ’s mini-exon B. The findings are especially relevant given the growing evidence that disruptions in microexon splicing may underlie several neuropsychiatric conditions. Conditions like autism and ADHD have been increasingly linked to impaired synaptic development, and this study helps explain one mechanism by which that might happen. It also highlights the need to study not just genes themselves but the tiny variations in how they’re assembled by the cell’s machinery. Looking ahead, these insights could inform the development of therapies that target splicing regulation or help restore normal synaptic balance in affected individuals. The research was conducted in collaboration with KAIST, KBSI, KISTI, Kyungpook National University, and Yonsei University. It was published in Nature Communications on May 13, 2025. 그림 설명
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS) |
| before |
|---|
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20