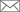주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
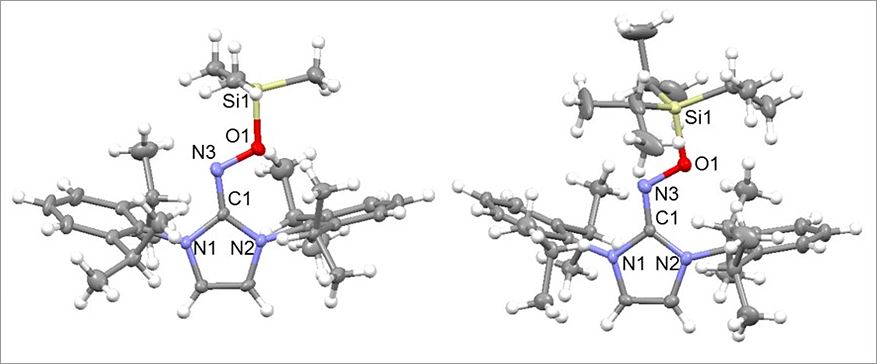
Catching Radical Molecules Before They Disappear- IBS researchers managed to stabilize short-lived radical ions which could be used for rechargeable batteries - While in most molecules, each electron finds a partner to pair up with, some electrons in radical molecules are left alone and unpaired. This configuration grants radicals with some unusual and interesting properties, which disappear as soon as the radicals react or interact with other molecules. It has been difficult to generate relatively stable radicals, because they react and change in the blink of an eye, but researchers from the Center for Self-Assembly and Complexity, within the Institute for Basic Science (IBS, South Korea) succeeded in synthesizing four new kinds of stabilized radicals. Unlike other molecules, some radicals have aligned spins, which confers them ferromagnetic properties, meaning that they can be attracted by a magnetic field. Due to these peculiar properties, radicals are likely to find applications in various fields, such as rechargeable batteries, molecular spintronics, and molecular magnetism. IBS scientists developed a strategy to stabilize oxime radicals, using N-heterocyclic carbenes (NHCs), as the latter can share their electrons to stabilize the unpaired electrons of the radicals. This result is particularly interesting as organic radicals are known to be very difficult to synthesize because they are more unstable than metal-containing radicals.
The radical structures were confirmed by single crystal X-ray diffraction analysis at Pohang Accelerator Laboratory and their properties were verified by electron paramagnetic resonance. The experimental results agreed well with the density functional theory. The same research group has also recently stabilized triazenyl radicals and used them as cathode materials for rechargeable lithium ion batteries. In the future, the researchers are taking up the challenge of producing more radical chemicals that have yet to be synthesized. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Self-assembly and ComplexityPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20