주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Peeling Graphene from Copper | ||
|---|---|---|---|
| Embargo date | 2016-08-30 17:00 | Hits | 2847 |
| Research Center |
Center for Multidimensional Carbon Materials |
||
| Press release | |||
| att. | |||
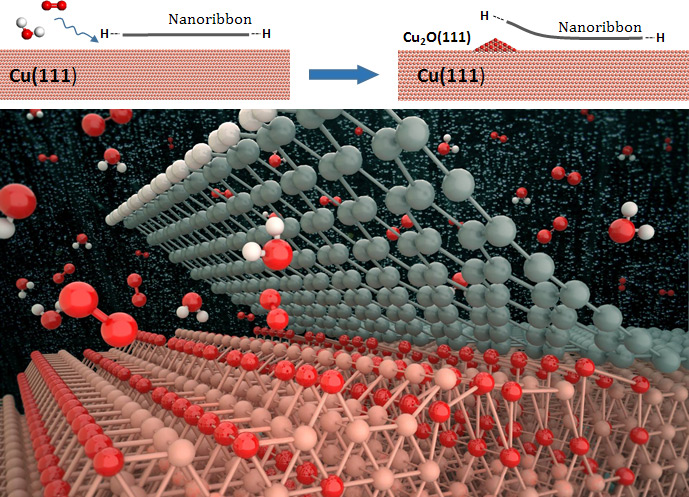
Peeling Graphene from Copper- Elucidating the intercalation mechanism and pathways for graphene decoupling from the copper substrate - August 30, 2016 A recent quantum chemistry study related to delamination of graphene from Cu foil substrates by a research team at the Center for Multidimensional Carbon Materials, Institute for Basic Science (IBS) and the Ulsan National Institute of Science and Technology (UNIST) has provided important information about the intercalation mechanism and pathways for graphene decoupling from the copper substrate through the formation of copper oxide, which has a weaker binding to the graphene than the pure Cu does.
The graphene films, grown on the copper (Cu) substrates must be detached clean without leaving residue behind, as residual metallic impurities can significantly alter electronic and electrochemical properties of graphene. However, thanks to recent advances in graphene transfer method, the electrochemical corrosion of graphene coatings on Cu has allowed the monolayer-thick material to be mechanically delaminated without significantly compromising its structural integrity. The new UNIST graphene research veers off in a new direction by succesfully separating graphene from its metal growth substrates without the assistance of the adhesive tape. The research findings have been published in the August issue of the Journal of the American Chemical Society (JACS). In the study, led by Prof. Sang Kyu Kwak (School of Energy and Chemical Engineering) and Prof. Rodney Ruoff (Center for Multidimensional Carbon Materials), the research team revealed the surface oxidation chemistry of the nanoribbon-covered Cu(111) surface. Specifically, they have demonstrated that the graphene nanoribbon (GNR) edge type influences the initial oxidation stages of the Cu surface, thus driving the nanoribbon decoupling by the intercalation of surrounding adsorbate molecules (e.g. oxygen and water). The difference between the armchair GNR and zigzag GNR on the Cu(111) substrate, is distinguished by the presence of an edge state in the zigzag GNR edges, which has been attributed to the hybridization between the out-of-plane carbon π orbitals and the metal d orbitals. This edge state, however, is absent in the armchair GNR edge atoms. Such an observation has not been reported for H-terminated GNR on Cu(111). Vibrational stretching mode calculations showed that the GNR edges influenced the molecular adsorption of oxygen at the bare and GNR/Cu sites, confirming the role of GNR edges in weakening the pre-elongated O-O bond at the GNR/Cu interface. The research team also explained that the GNR edges facilitated the stabilization of water molecules (regardless of surface oxygenation), which would otherwise be unstable on the bare Cu surface.
Dr. Kester Wong, who takes full charge of the research, notes that "GNR-mediated interactions between water and the chemisorbed oxygen radicals can shed further light in elucidating the role of water and oxygen in the surface oxide formation." "This particular study may have interesting implications for the development of regioselective graphene-based catalysis. Nonetheless, employing other crystal facets for the interfacial study of low dimensional materials is of great interest, and several investigations are being heavily pursued by our group in this area", says Prof. Kwak. This work has been supported by Institute of Basic Sciences (IBS, CMCM) and Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korean Ministry of Science, ICT & Future Planning (MSIP). Joo Hyeon Heo (UNIST, Public Relations Team) Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
|||
| before | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20