주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School

Biomedical Mathematics GroupMathematicians Tame Cellular “Noise” to Control Life at the Single-Cell LevelResearchers develop a “Noise Controller” that solves a decades-old biological puzzle, paving the way for precision cancer therapy and synthetic biologyRead More

Center for Neuroscience Imaging ResearchNew Bioelectronics Device Based on Hydrogel- Elastomer Conductive NanomembranesTransformable and Imperceptible Nanomembranes Enable Seamless Integration of Electronics with Living TissueRead More

Center for Exotic Nuclear StudiesScientists Discover New Nuclear “Island” Where Magic Numbers Break DownResearchers discover an “Island of Inversion” in proton-neutron symmetric nuclei for the first timeRead More

Center for Theoretical Physics of Complex SystemsNew Theory Explains the True Upper Limit of Conventional SuperconductivityA new theoretical study has identified a fundamental kinetic barrier that limits how strongly electrons can couple to lattice vibrations in metals. Because electron–phonon coupling directly controls the superconducting critical temperature in conventional superconductors, this finding places a strict upper limit on how high the critical temperature can rise in phonon-mediated systems. The work was supported in part by the Institute for Basic Science (IBS) in Korea.Read More

Center for Theoretical Physics of Complex SystemsIBS Researchers Reveal New Light-Matter Interaction in Time-Modulated Photonic MediaA research team including scientists at the Institute for Basic Science (IBS) in South Korea has uncovered a groundbreaking way for atoms to emit and absorb light using time-periodic photonic structures, known as photonic time crystals (PTCs). Their findings challenge the conventional understanding of light-matter interaction and reveal a new physical process called spontaneous emission excitation, where atoms absorb energy from temporal modulation while emitting light. This phenomenon is impossible in static environments.Read More
Featured
 2026-01-02Mathematicians Tame Cellular “Noise” to Control Life at the Single-Cell Level
2026-01-02Mathematicians Tame Cellular “Noise” to Control Life at the Single-Cell Level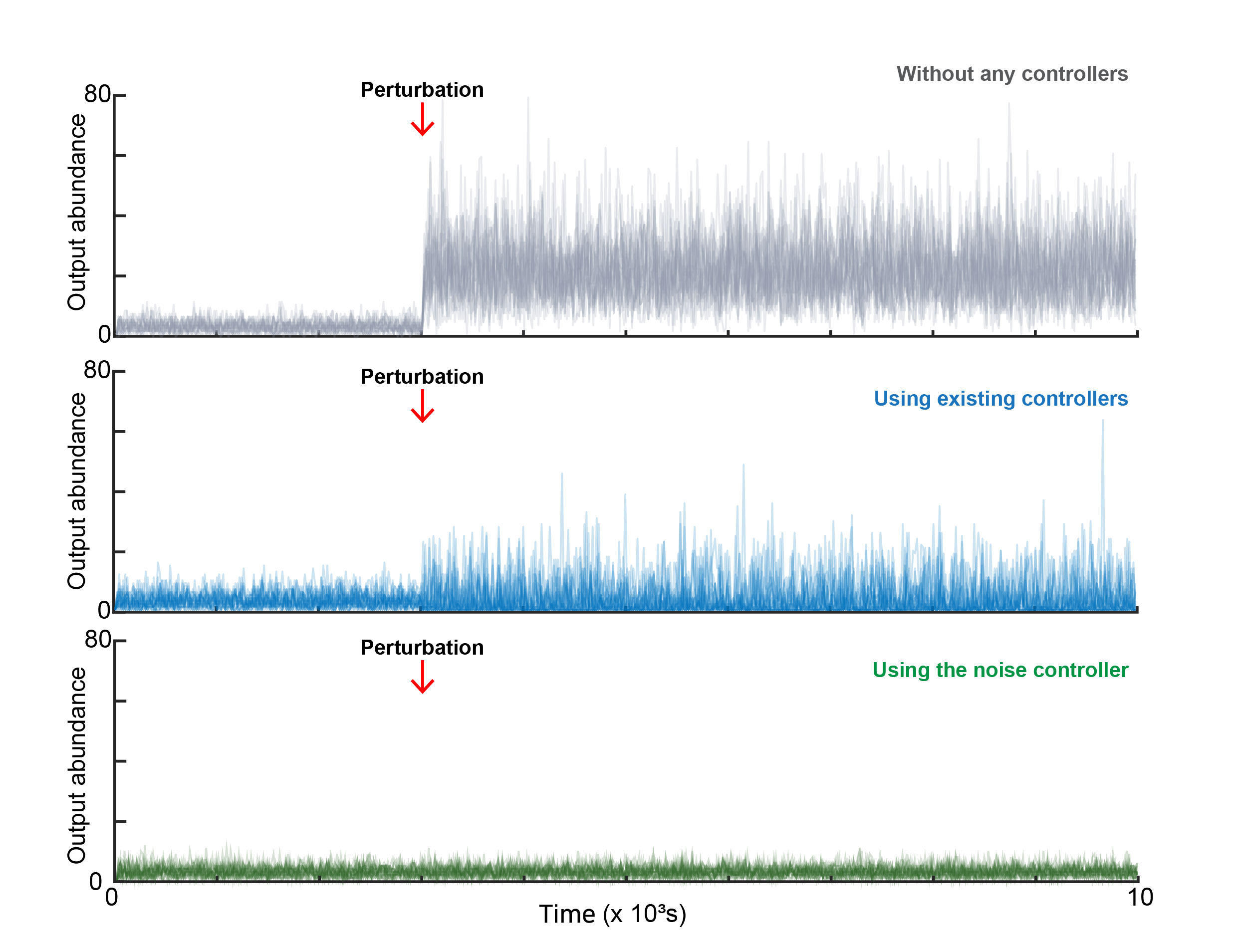
 2025-12-24Researchers Discover How Stomach Cancer Learns to Grow on Its Own
2025-12-24Researchers Discover How Stomach Cancer Learns to Grow on Its Own
 2025-12-10New Bioelectronics Device Based on Hydrogel- Elastomer Conductive Nanomembranes
2025-12-10New Bioelectronics Device Based on Hydrogel- Elastomer Conductive Nanomembranes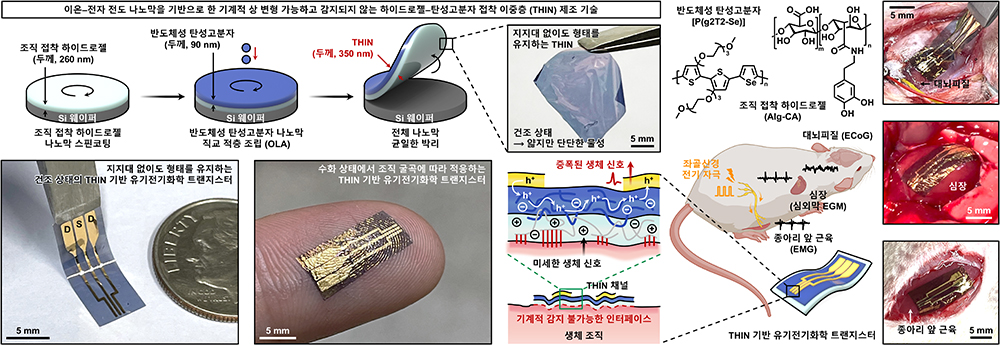
 2025-12-08Scientists Discover New Nuclear “Island” Where Magic Numbers Break Down
2025-12-08Scientists Discover New Nuclear “Island” Where Magic Numbers Break Down
 2025-11-18New Theory Explains the True Upper Limit of Conventional Superconductivity
2025-11-18New Theory Explains the True Upper Limit of Conventional Superconductivity
 2025-11-17IBS Researchers Reveal New Light-Matter Interaction in Time-Modulated Photonic Media
2025-11-17IBS Researchers Reveal New Light-Matter Interaction in Time-Modulated Photonic Media
 2025-11-05More Polar Ocean Turbulence due to Planetary Warming
2025-11-05More Polar Ocean Turbulence due to Planetary Warming
 2025-10-16Climate Whiplash Effects Due to Rapidly Intensifying El Niño Cycles
2025-10-16Climate Whiplash Effects Due to Rapidly Intensifying El Niño Cycles
 2025-10-04Tiny sugars in the brain disrupt emotional circuits, fueling depression
2025-10-04Tiny sugars in the brain disrupt emotional circuits, fueling depression
 2025-09-25Robots Map Chemical Reaction “Hyperspaces” to Unlock Complex Networks
2025-09-25Robots Map Chemical Reaction “Hyperspaces” to Unlock Complex Networks
 2025-09-24Running Dry – A New Study Warns of Extreme Water Scarcity in the Coming Decades
2025-09-24Running Dry – A New Study Warns of Extreme Water Scarcity in the Coming Decades
 2025-09-22IBS Researchers Directly Demonstrate Unusually Strong Magnon-Phonon Coupling in Kagome Antiferromagnet Mn3Ge
2025-09-22IBS Researchers Directly Demonstrate Unusually Strong Magnon-Phonon Coupling in Kagome Antiferromagnet Mn3Ge
 2025-08-22Hemoglobin Reimagined: A Breakthrough in Brain Disease Treatment
2025-08-22Hemoglobin Reimagined: A Breakthrough in Brain Disease Treatment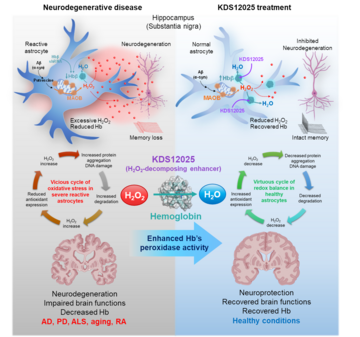
 2025-08-12Mirror-Like Graphite Films Break Records in Strength and Conductivit
2025-08-12Mirror-Like Graphite Films Break Records in Strength and Conductivit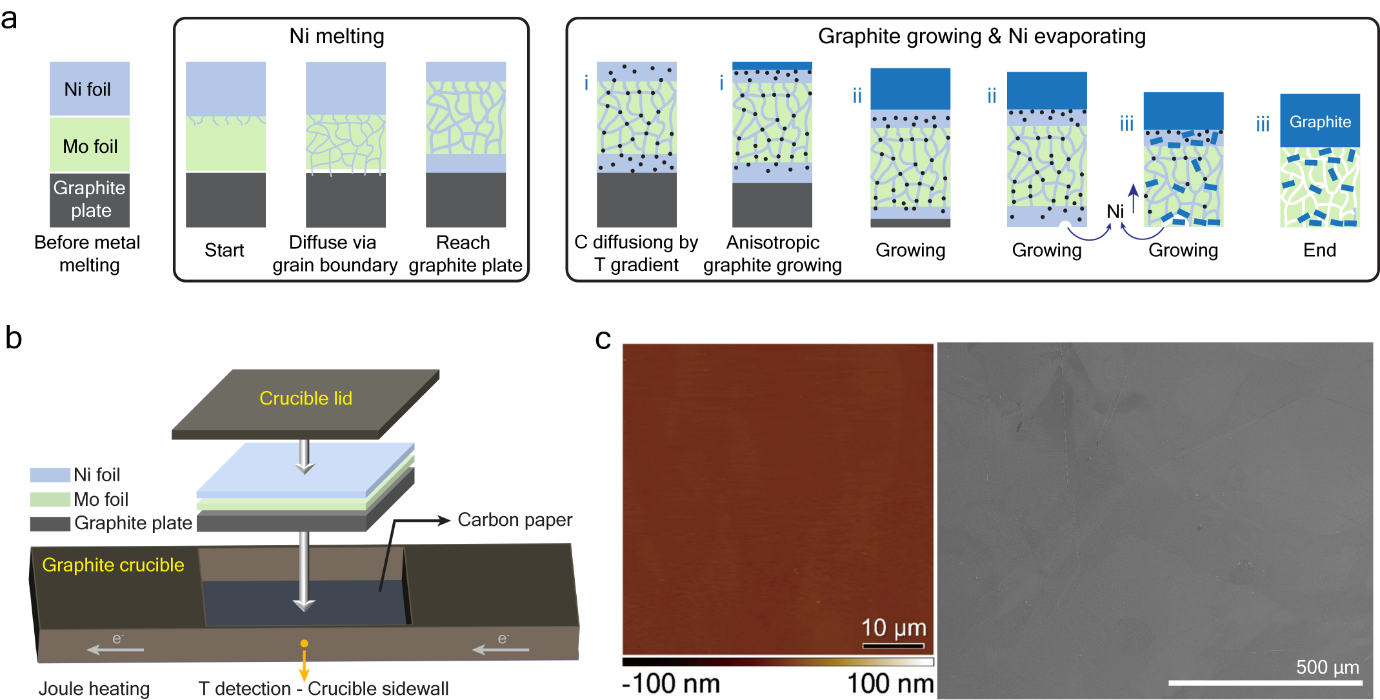
 2025-08-06IBS Researchers Uncover Major Flaw in Popular Method for Modeling Quantum Chaos
2025-08-06IBS Researchers Uncover Major Flaw in Popular Method for Modeling Quantum Chaos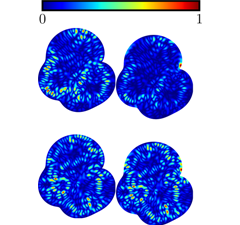
 2025-07-29Astrocytes Identified as Hidden Culprit Behind PTSD
2025-07-29Astrocytes Identified as Hidden Culprit Behind PTSD
 2025-07-21How the Brain Controls Its Blood Volume
2025-07-21How the Brain Controls Its Blood Volume
 2025-06-12Capturing the Fleeting Transformation of Perovskite Nanomaterials Under Light
2025-06-12Capturing the Fleeting Transformation of Perovskite Nanomaterials Under Light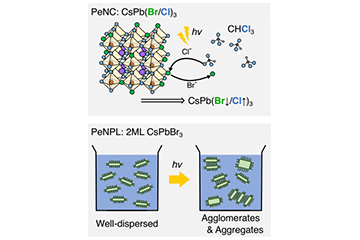
 2025-06-11From Plastic Waste to Clean Hydrogen: A Scalable Solar-Powered Solution
2025-06-11From Plastic Waste to Clean Hydrogen: A Scalable Solar-Powered Solution
 2025-06-05New Non-Invasive Method Discovered to Enhance Brain Waste Clearance
2025-06-05New Non-Invasive Method Discovered to Enhance Brain Waste Clearance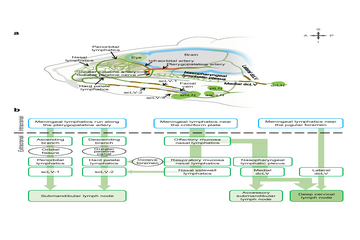
 2025-05-22Tiny Genetic Switch Found to Control Brain Balance and Behavior
2025-05-22Tiny Genetic Switch Found to Control Brain Balance and Behavior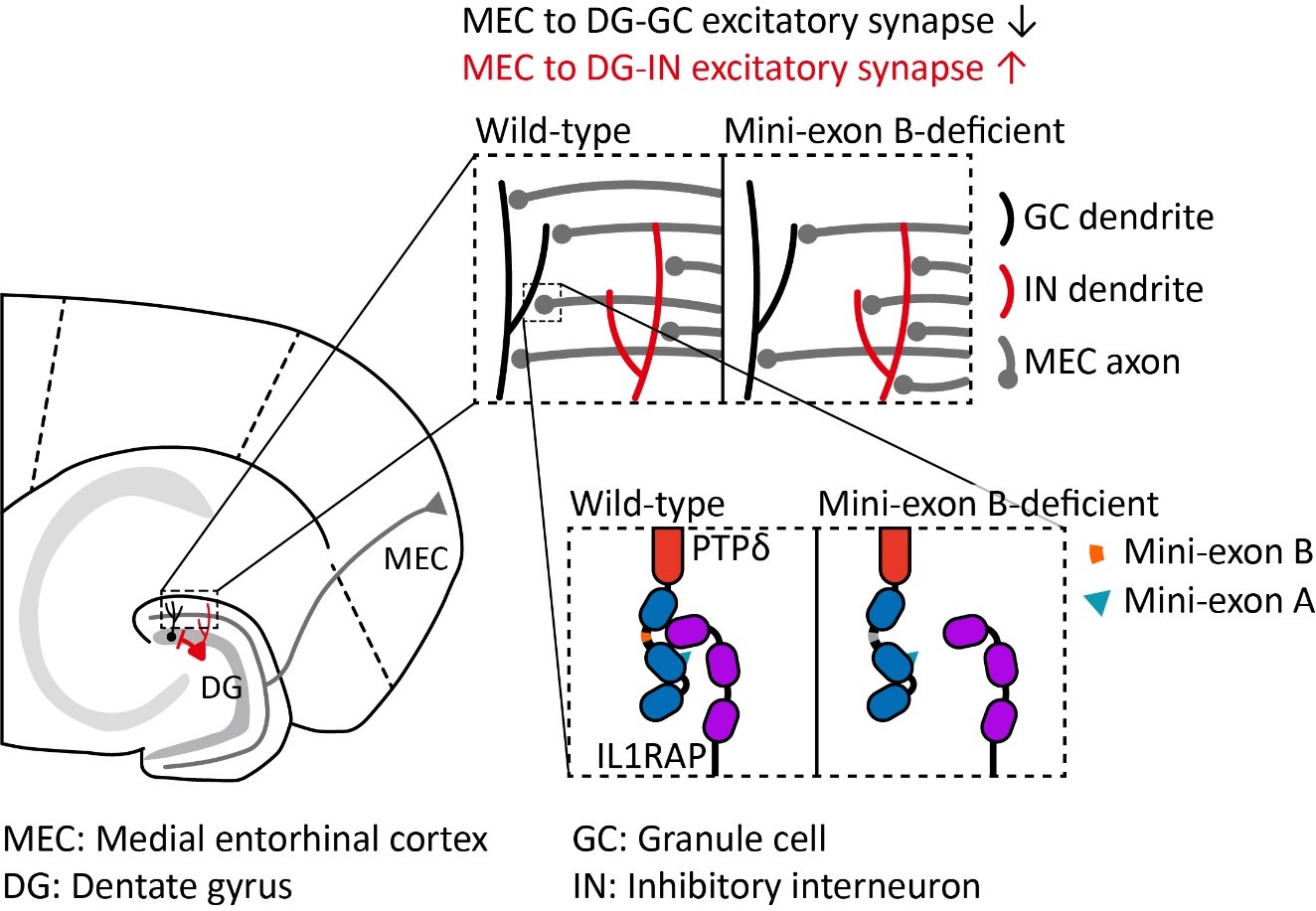
 2025-05-20“Cutting to Survive”: How Cells Remove DNA Bridges at the Last Moment
2025-05-20“Cutting to Survive”: How Cells Remove DNA Bridges at the Last Moment
 2025-05-16World’s Largest Bat Organoid Platform Paves the Way for Pandemic
2025-05-16World’s Largest Bat Organoid Platform Paves the Way for Pandemic
 2025-04-22Brain-Inspired AI Breakthrough: Making Computers See More Like Humans
2025-04-22Brain-Inspired AI Breakthrough: Making Computers See More Like Humans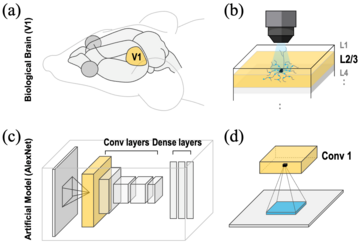
 2025-04-14Scientists Identify Key Enzyme in Alzheimer’s Disease That Links Brain Inf
2025-04-14Scientists Identify Key Enzyme in Alzheimer’s Disease That Links Brain Inf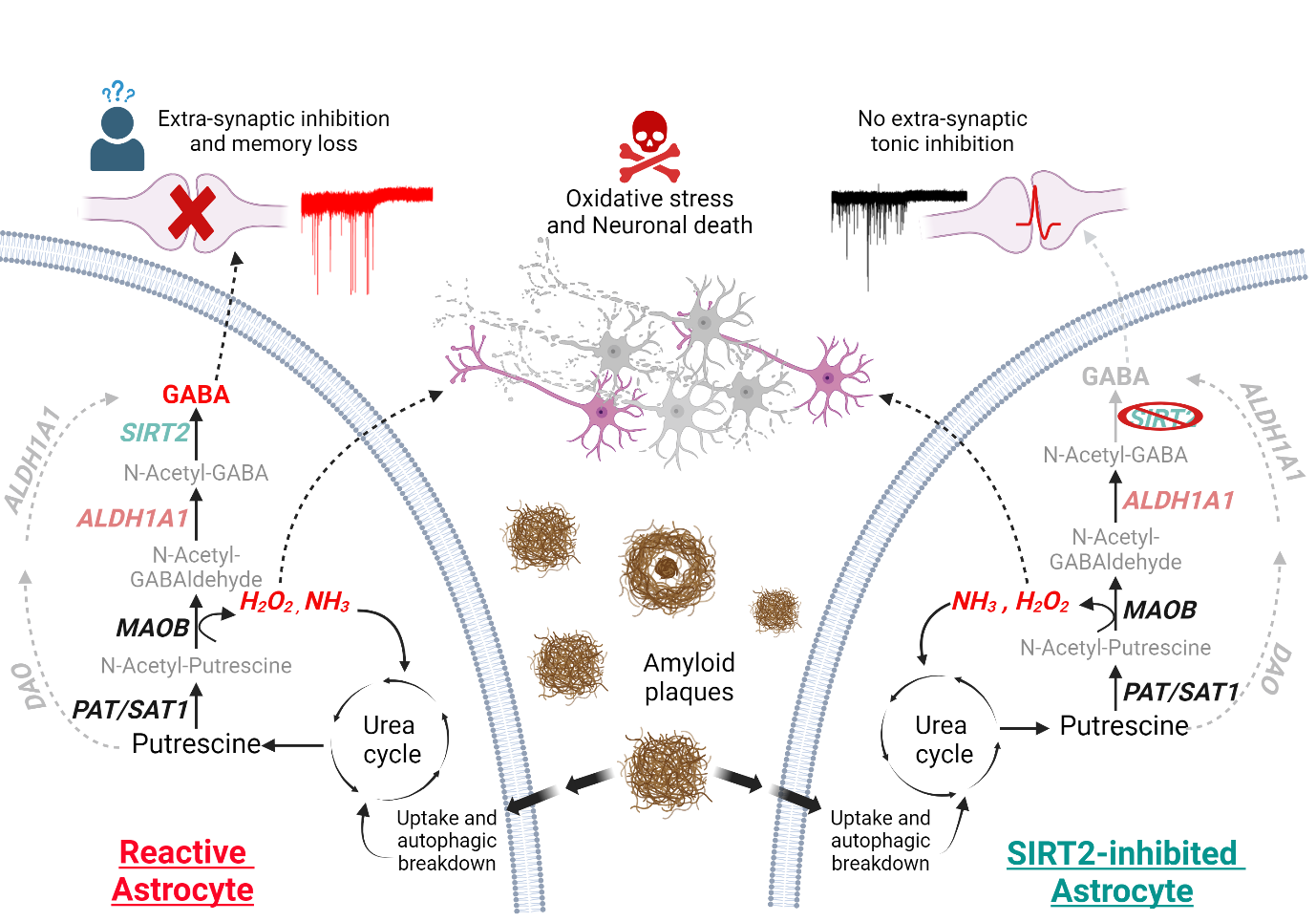
 2025-04-04Cellular regulator of mRNA vaccine revealed... offering new therapeutic options
2025-04-04Cellular regulator of mRNA vaccine revealed... offering new therapeutic options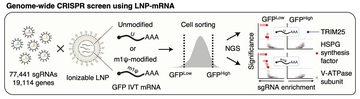
 2025-03-31A Neural Compass for Fear: Mapping How the Brain Distinguishes Between Direct and Vicarious Fear
2025-03-31A Neural Compass for Fear: Mapping How the Brain Distinguishes Between Direct and Vicarious Fear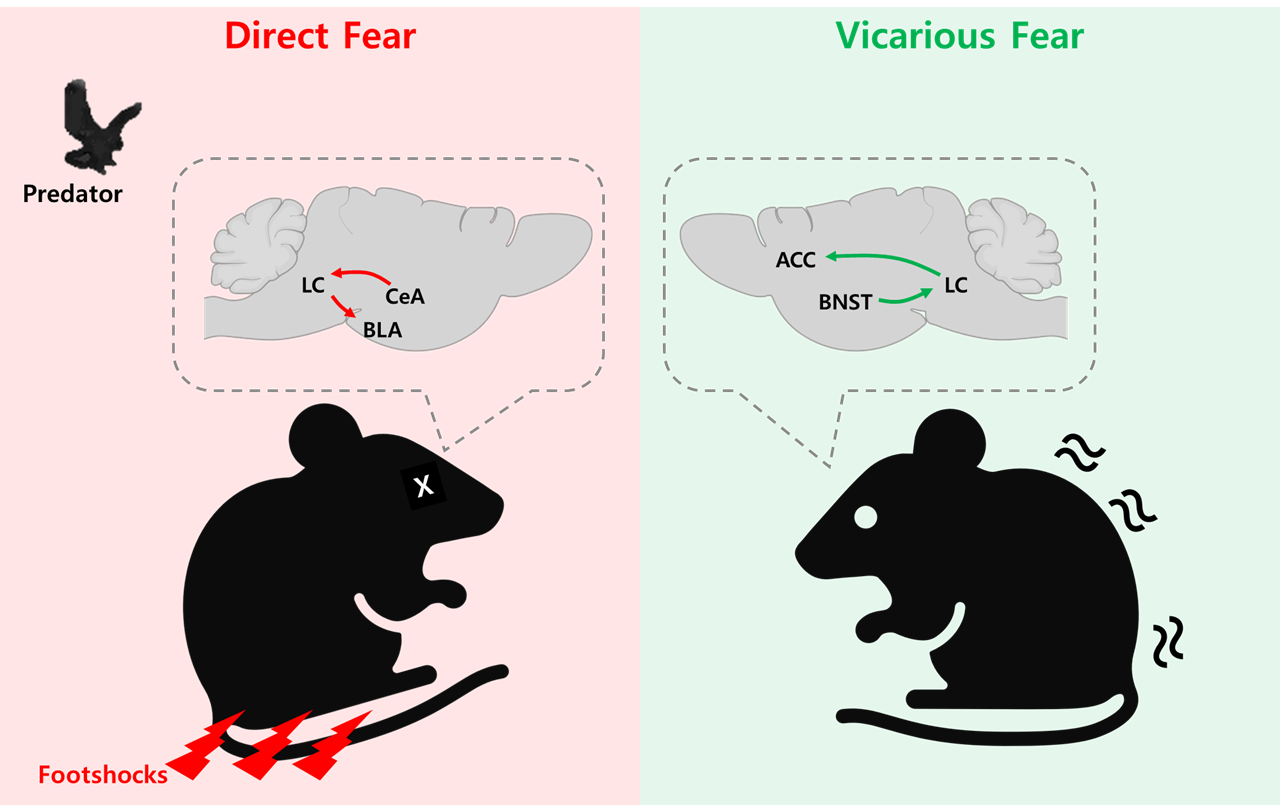
 2025-03-26How Movement Affects the Way the Brain Processes Sound and Sight
2025-03-26How Movement Affects the Way the Brain Processes Sound and Sight
 2025-02-03How Does the Hippocampus Coordinate Memory Encoding and Retrieval?
2025-02-03How Does the Hippocampus Coordinate Memory Encoding and Retrieval?
Job Board
read more- 2026-1 IBS Center for Algorithmic and Robotized Synthesis Open Recruitment In progress
- 2026-1 Recruitment Announcement for Research Positions at the Center for Trapped Ion Quantum Science In progress
- 2026-1 Recruitment Announcement for Postdoctoral Research Associate Position at the Center for Quantum Conversion Research In progress
- 제2026-1회 기초과학연구원 지하실험 연구단 연구기술직 채용 공고 In progress
Research CentersOpenclose
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures

















