주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
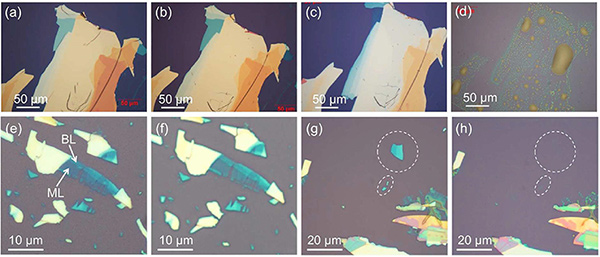
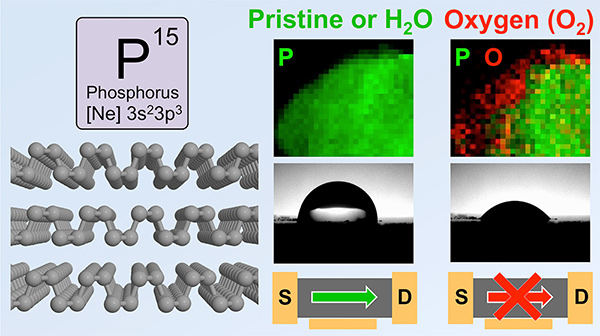
Black Phosphorus Doesn't Mind Water (if it is de-aerated)- IBS researchers disprove the idea that water degrades black phosphorus and find the material is actually hydrophobic - Researchers at the Center for Multidimensional Carbon Materials (CMCM), within the Institute for Basic Science (IBS) have discovered that one of graphene's competitors, black phosphorus, is inert to water deprived of oxygen, ending the debate of whether water causes its degradation. Their research, accepted by Chemistry of Materials, provides a more complete understanding of the role of molecular oxygen and water in the degradation of black phosphorus. Black phosphorus is a 2D material structurally similar to graphene with extraordinary electrical and optoelectric properties. However, unlike graphene, black phosphorus has the advantage of having a tunable bandgap. A bandgap is an energy barrier, essential for controlling the flow of electrons, like an on/off switch. Black phosphorus' bandgap varies depending on the number of black phosphorus layers: The more layers, the smaller the bandgap. This makes it interesting for the next generation nanoelectronic and photoelectronic devices. However, the perceived instability of black phosphorus to oxygen and water was never before been carefully addressed. The IBS team questioned the previous thinking on the degradation mechanisms of black phosphorous. Researchers tested samples of black phosphorous under a number of different conditions. They checked if there is a difference between water that contains air and de-aerated water. They found that the physical and electronic properties of samples stored in de-aerated water did not degrade.
To further clarify the role of oxygen in the degradation of black phosphorus, IBS researchers performed experiments with different oxygen isotopes (oxygen-18 and oxygen-16). They used gas with oxygen-18 and water with oxygen-16, so they could distinguish if the damage was caused by oxygen, water or both. The results confirmed that it is not water, but rather oxygen, that reacts with black phosphorous. Furthermore, IBS researchers discovered that the surface of black phosphorous is actually hydrophobic, in contrast to previous experiments. "It was previously thought that water could react with black phosphorous, but thanks to these experiments, we can be reassured that it is oxygen, not water, that damages black phosphorus," notes Prof. Rodney Rouff, CMCM director and corresponding author.
"Water by itself does not cause any damage because it is just absorbed by the surface of black phosphorus flakes as an intact molecule. Oxygen, instead, dissociates into two oxygen atoms and oxidizes the flake. Once the material is oxidizes by oxygen, water absorbs stronger, transforming the material from hydrophobic to hydrophilic," explains Ruoff. In summary, water (deprived of oxygen) causes only small changes due to the fact that it is difficult to get rid of all oxygen in our environment, but oxygen substantially modifies the electronic structure of this 2D material and accelerates its degradation. These results could open new pathways for exploring applications that require contact with aqueous solutions such as: Solution gating; electrochemistry; and solution-phase approaches for exfoliation, dispersion, and delivery of black phosphorus. Most of the experiments were performed by Dr. Yuan Huang (CMCM, IBS), in collaboration with colleagues at a number of institutions, including Prof. Peter Sutter (University of Nebraska). Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Suh, William Insang 042-878-8137
- Last Update 2023-11-28 14:20