Looking into the “micro universe” with ultrafast lasers
The IBS Center for Relativistic Laser Science captures multiple molecular orbitals
At last, the “celestial body of misfortune” made its appearance. As if out of loyalty to the country that first discovered it about 80 years ago, Pluto, through the New Horizons spacecraft sent by the U.S. National Aeronautics and Space Administration (NASA), proudly confirmed to humanity that its diameter is 70~80 km bigger than that of Eris, a dwarf planet which had been decisive in degrading Pluto from the position of a planet to a dwarf planet.
On the night of July 14, 2015, scientists and many others who yearn to know the secrets of the universe were waiting for Pluto’s upclose images to be disclosed by New Horizons for the first time in human history. They may have sought the enticing thrill, albeit for a short moment, provided by the piquant experience of identifying the edge of our vast solar system which is inaccessible to the naked eye.
It may be somewhat unfair to regard the yearning for knowledge of a world that is unknown, invisible, intangible, and unreachable as simple curiosity. Rather, that yearning may be based on the “unwavering pride” of humanity and science. Ambitious explorations are conducted not only into the boundless space beyond the Earth, but also into the smallest spaces that surround us: the micro world inside molecules.
Imagining the track movement of electrons Laser facilities at GIST. The IBS Center for Relativistic Laser Science is using femtosecond lasers to probe the micro world of molecules. Laser facilities at GIST. The IBS Center for Relativistic Laser Science is using femtosecond lasers to probe the micro world of molecules.
Scientists have long imagined that a molecular structure may be similar to a solar system in a sense. In our solar system, the Sun is in the center and planets revolve around the Sun, following their own orbits. Similarly, atoms that make up a molecule have been thought to have a nucleus in the center, around which electrons follow their own orbits. The difference lies in that while planets orbit in a defined “plane,” molecular orbitals are indefinite and “three dimensional.”
The time scales in a solar system and in a molecular structure are completely different as well. In a solar system, a period of time may be regarded as an instant; inside a molecule, however, the time period may be treated as if it were eternity. In the blinking of an eye, electrons repeat the same movement numerous times, during which their energy states change incessantly.
In order to just roughly track electrons which refuse to hold still like a fidgety child, scientists came up with a hypothesis that if a solution to the Schrödinger equation allows an electron to have a minimum of energy, a space satisfying such a solution would be the electron’s orbit. In other words, the orbit has an electron which has the least necessary energy to exist inside a molecule. The shapes of molecular orbitals vary depending on the location and energy state of individual electrons. Imagine that a molecule is composed of orbitals that resemble clouds of varying shapes. Ever since it was first posited, scientists have continuously tried to determine whether this hypothesis is true or false.
Whenever an electron’s location or energy changes, the physical and chemical properties of a molecule that has the electron change as well. For example, the chemical reaction of intermolecular bond breaking or bond formation depends on the ultrafast movement of atoms in the molecules. Some reactions occur easily, while others do not seem to happen at all; some reactions occur instantly, while others are done at a snail’s pace.
Here, let’s make a daring assumption. If we can observe in detail or even predict molecular orbitals, couldn’t we manipulate chemical reactions by ourselves? Although techniques for observing an object as small as a molecule are available, techniques that can identify electrons’ irregular movement have not been developed until recently.
Lasers transform the dreams of scientists into reality
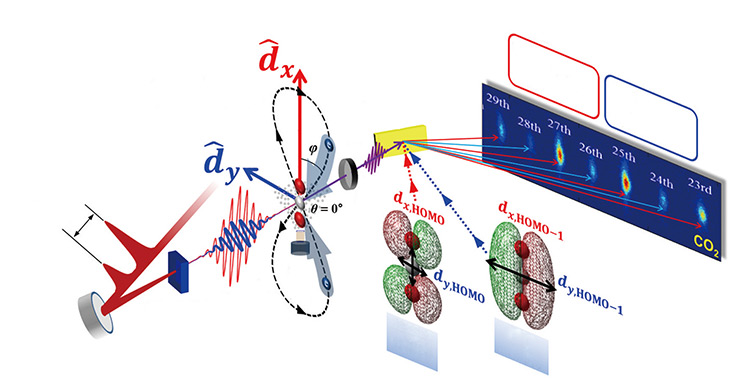 Schematic layout for twodimensional high-harmonic spectroscopy for probing multiple molecular orbitals. The spectral image shows a typical harmonic spectrum from aligned CO2 molecules. The HOMO and HOMO-1 can be separately encoded in the odd and even harmonics by the different strength of dipole moment induced in the two-color laser field on the two orthogonal axes. Schematic layout for twodimensional high-harmonic spectroscopy for probing multiple molecular orbitals. The spectral image shows a typical harmonic spectrum from aligned CO2 molecules. The HOMO and HOMO-1 can be separately encoded in the odd and even harmonics by the different strength of dipole moment induced in the two-color laser field on the two orthogonal axes.
A microscope is the representative tool for observing an object invisible to the naked eye.
However, this may no longer be the case for modern scientists: they have recently turned to lasers for looking into the space inside molecules. The lasers used for the observation of a micro universe are different from the ordinary ones which decorate the night sky with colorful patterns at a festival. In 2004, a Canadian research group discovered the ability of special lasers to “see” an infinitesimal world.
When a strong laser field with a specific wavelength is applied to aligned molecules in order to observe molecular orbitals, electrons escape their orbitals, making the orbitals become ionized. In the process of the electrons’ returning to their place, the orbitals emit scattered waves (high harmonic radiation) of different frequencies, which contain information about the orbitals.
The frequency of high harmonic radiationfollows a rule of increasing by a multiple of certain numbers. The Canadian research group realized that it was possible to understand the characteristics of molecular orbitals by analyzing high harmonic radiation.
At that time, the research group used lasers to observe nitrogen (N2) molecules. Through high harmonic generation (HHG), they successfully reconstructed the images of the highestoccupied molecular orbital (HOMO) of N2. The outcomes gave hope to scientists that they might be able to see other electrons deeper inside an atom. However, it was not an easy task. In order to generate the high harmonics that contain an electron’s orbital information, the electron must escape its orbital. Depending on the location and the distance (from the nucleus) of each orbital, different energies are required for ionization.
It was the IBS Center for Relativistic Laser Science that recently suggested, to the low-spirited scientific community, new possibilities with a novel idea: a “pincer movement” tactic involving the use of a two-color (two-wavelength) laser field.
The possibility of capturing the moment of chemical reactions
The IBS Center’s researchers had laser pulses pass through a special material, which split the pulses into two different waves. Then, the researchers polarized the waves so that they could be perpendicular to each other when applied to aligned CO2 molecules. While the Canadian research group tried a single-color laser field on a one-dimensional plane, the IBS Center used two-dimensional high harmonic spectroscopy (HHS). When the orthogonally polarized waves passed through the CO2 molecules, multiple high harmonic radiations were emitted from the molecules when electrons escaped from and returned to their orbitals.
The two-color laser field produced odd and even harmonics. This means that two kinds of harmonic radiations were generated, relative to using a single-color laser field. Moreover, it was discovered that different orbitals could be separately characterized by observing odd and even harmonics. To be specific, the characteristics of the HOMO and the HOMO-1 (a lower lying molecular orbital) were projected into odd and even harmonics, respectively. According to the IBS Center, it was the first experiment to successfully observe more than one molecular orbital separately by using the HHS. The Center’s outcomes were the world’s first proof that lasers, despite their limitations, can be used to track multiple molecular orbitals.
The results were published in the online edition of Physical Review Letters (dated April 14, 2015), an international physics journal.
Ahead of the IBS Center’s achievement, about four years ago, Nature published a paper which proved that one-dimensional high harmonic radiation could capture the moment in which a bromine (Br) molecule split into atoms during chemical reactions. In other words, the authors of the paper succeeded in tracking exactly when the molecule-atom transition occurred in the process of chemical reactions. The IBS Center’s research outcomes have increased the expectations among people in and out of the scientific community that the two-dimensional HHS could help researchers identify not only the moment of the molecule-atom transition, but also the characteristics of various other moments in which chemical reactions occur.
From 1 quadrillionth of a second to 1 quintillionth of a second
 Beginning his research on femtosecond lasers in the mid-1980s, Director Nam has led the “localization” of laser technology. He has been directly involved in making laser devices such as spectroscopes and resonators. Beginning his research on femtosecond lasers in the mid-1980s, Director Nam has led the “localization” of laser technology. He has been directly involved in making laser devices such as spectroscopes and resonators.
In the micro world of molecules, changes that occur in an extremely short time frame can be captured by a femtosecond laser, a special light that moves at an ultrafast speed uncommon in our macro world. The speed of the lasers used by the researchers at the IBS Center was 30 femtoseconds, or 30 quadrillionths of a second. Lasers slower than that cannot dare to dream about being used in tracking chemical reactions on a molecular level.
Scientists nowadays seem to be losing interest in femtosecond lasers. Instead, they seem to be looking for attosecond lasers as another object of “pride” for science. Of course, it is sufficient to use femtosecond lasers for observing the orbitals of a molecule made up of many atoms or a multi-molecular compound. However, you need much faster lasers in order to understand how electrons move inside an atom. An attosecond means an unimaginably fast time frame of 1 quintillionth of a second.
While some of the femtosecond laser technologies are commercially available, attosecond lasers should go a long way toward being widely used in research. Attosecond laser pulses can be generated by using femtosecond laser pulses. In fact, it is very difficult to deal with femtosecond lasers as well. As most of the devices for generating femtosecond laser pulses are very sensitive to even minuscule environmental changes, they must be “taken good care of ” in a clean room, where consistent temperature and humidity levels are maintained. The sensitiveness of the devices accounts for the great difficulties involved in the generation of appropriate laser pulses. Whether an experiment succeeds or not on a given day largely depends on the state of the devices and the lasers on that day.
For example, humidity can easily damage the surface of a mirror used for femtosecond lasers. The mirrors designated for these processes are manufactured in a way that meets the requirements of desired wavelengths. It is impossible to generate femtosecond lasers by using the ordinary mirror that we use every day. Although such a mirror seems to reflect all the light that it comes in contact with, it loses a considerable amount of light particles as well as the energy they contain, which is why lasers from an ordinary mirror would cause inaccurate results. When asked why he seeks to observe the micro world by “currying favor” with lasers, Chang Hee Nam, professor at the Department of Physics and Photon Science at the Gwangju Institute of Science and Technology (GIST) and Director of the IBS Center for Relativistic Laser Science, explains, “To discover a physical phenomenon that existing science and technology have not been able to find, and to replicate in a laboratory extreme phenomena that take place either on a cosmic or molecular scale.” Beginning his research on femtosecond lasers in the mid-1980s, Director Nam has led the “localization” of laser technology. He has been directly involved in making laser devices such as spectroscopes and resonators. Director Nam recalled, “Because the field had never been researched in Korea, our project underwent many twists and turns during its initial stages. The students have contributed a lot with their ideas and efforts.”
Coincidentally, this year marks the International Year of Light, as designated by the United Nations Educational, Scientific and Cultural Organization (UNESCO). Director Nam’s research group is developing “unusual lights” in its honor. His group is convinced that someday, the lights will enable researchers to thoroughly probe the structure of molecules and atoms that still remain largely unknown.
Written by
So-Hyeong Lim | Journalist for the Hankookilbo
|
















