Hydrocarbons are naturally abundant on the earth and exist in
various types. They can offer useful substrates for many chemical
synthesis, such as the development of new drugs or materials, but
directly converting hydrocarbons into more value-added products has
been highly limited by their inactivity and difficulties associated with
selectivity: their very inactive scaffold bonds, carbon-hydrogen bonds,
require great amounts of energy to break and the selective activation
of a specific bond is extremely difficult. While research efforts in this
area is reporting several advancements in developing more reactive
and selective chemical processes, the IBS Center for Catalytic
Hydrocarbon Functionalization (Director Sukbok Chang) (has recently
succeeded in developing a robust carbon-hydrogen bond amidation
process, making great strides for the hydrocarbon processing industry.
Synthetic chemistry forms an important basis
for the pharmaceutical and materials industries.
It has been advancing to fulfill the growing
demand for technology that handles molecules
with a high precision and efficiency. A large
volume of research has been dedicated to
identifying and designing more efficient routes
to synthesize molecules.
One of the major breakthrough achievements
from such efforts was the employment of
transition metals as catalysts for organic
reactions. With the knowledge of reaction
mechanisms involving these various transition
metals, we are now able to break many strong
chemical bonds with a low amount of energy
and make bonds with high accuracy, once
considered impossible.
The Center for Catalytic Hydrocarbon
Functionalizations is one of the leading research
groups in catalytic reactions of transition
metals. Using these metal complexes as catalysts,
the Center has been conducting in-depth
research to directly install nitrogen atoms onto
hydrocarbons to produce amino compounds
that contain various nitrogen functional
groups. These amino compounds have an
industry-wide application for products such as
medical, electronic and optical materials, but
directly converting hydrocarbons into amino
compounds has been a challenge for a long
time. The most commonly employed technique
is a complex process because hydrocarbons
need to be pre-functionalized before nitrogen
atoms are introduced. A great deal of recent
research has focused on simplifying the
process, resulting inmore efficient routes to
functionalize hydrocarbons. The Center has
recently succeeded in developinghighly stable
and commercially viable process to form amino
compounds.
In fact, back in 2012, the Center developed a
rhodium-based catalytic system where azides,
an amino source with three nitrogen atoms,
were adopted in order to activate hydrocarbons.
Though the system could produce amino compounds
that are widely applicable for various molecules
and was also environmentally friendly as
it releases nitrogen as a single by-product.
However, it was not without flaws: it needed
large amounts of expensive rhodium metals and
a high reaction temperature of over 80℃.
The Center started investigating the mechanisms
behind the rhodium-azide reaction and has
recently found why it requires a high rhodium
loading and an elevated reaction temperature.
When rhodium catalysts activate a hydrocarbon
to have nitrogen introduced, azide compounds
should form a covalent bond with the catalysts
by donating a pair of electrons. However, azide
functional groups are turned out to have weak
ability to coordinate with rhodium metals,
requiring a high catalyst loading and high
reaction temperature. In order to improve the efficiency of the process, a new amino source
with high coordination propensity had to be
found.
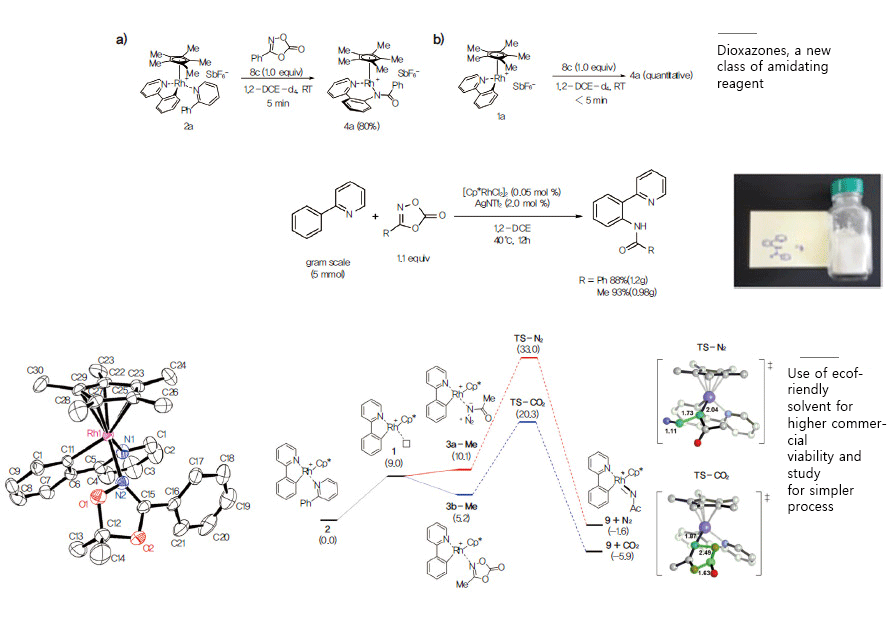
The Center set its eyes on dioxazolones which
have sp2 hybridized nitrogen atoms. As their
unshared electron pairs are easily activated with
oxygen and form stronger bonds with catalytic
metals, they seemed like a viable alternative to
azides.
Upon substituting azides with dioxazolones,
the Center found out, the same amidation
reaction takes place at room temperature with
amount of rhodium catalysts reduced by 60%.
This newly developed amidating reagent of
1,4,2-dioxazol-5-ones is applicable to a broad
range of substrates with high functional group
tolerance and only releases carbon dioxide as
a by-product. It is more convenient to prepare,
store and use compared with potentially explosive
azides and has a high commercial viability
because it uses ecofriendly ester as solvents.
With further research, the Center identified the
structure of the reaction intermediates and the
reaction mechanisms through computational
quantum chemistry and explained the higher
reactivity of the process. Its research was
recognized as a pioneering example of reaction
development on the basis of mechanistic study
and published in the Journal of the American
Chemical Society in March 2015. The practical
aspect of the research drew significant industrial
interest, and thus, the Center continued the
research, developing a large scale, ecofriendly
manufacturing process.
The Center is currently studying how to
replace expensive rhodium catalysts with lowpriced
transition metals to take a step closer
toward an ideal carbon-hydrogen amination
process. Its goal is to shorten the process so
that hydrocarbons can be activated in a highly
stable, economical and ecofriendly manner.
Published paper
Yoonsu Park, Kyung Tae Park, Jeung Gon Kim, and Sukbok Chang, “Mechanistic Studies on the Rh(III)-Mediated Amido Transfer Process Leading to Robust C−H Amination with a New Type of Amidating Reagent”, Journal of the American Chemical Society, Vol. 137, no. 13, pp. 4534–4542 (2015)
References
Ji Young Kim et al., “Rhodium-Catalyzed Intermolecular Amidation of Arenes with Sulfonyl Azides via Chelation-Assisted C–H Bond Activation”, Journal of the American Chemical Society, Vol. 134, no. 22, pp. 9110-9113 (2012)
Kwangmin Shin, Hyunwoo Kim, and Sukbok Chang, “Transition-Metal-Catalyzed C–N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C–H Amination”, Accounts of Chemical Research, Vol. 48, no. 4, pp. 1040-1052 (2015)














