주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Correction of a Faulty Gene in Human Embryos | ||
|---|---|---|---|
| Embargo date | 2017-08-03 02:00 | Hits | 3559 |
| Research Center |
Center for Genome Engineering |
||
| Press release |
 News Coverage.xls
News Coverage.xls
|
||
| att. | |||
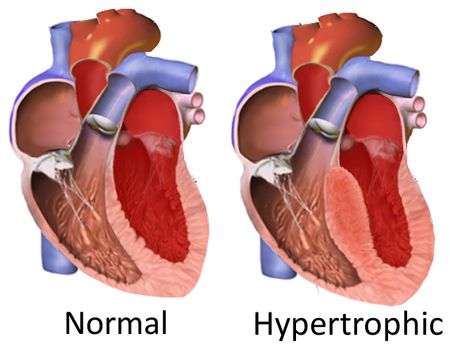
Correction of a Faulty Gene in Human Embryos- Published in Nature, CRISPR-Cas9 genetic scissors open up new pathways to treat and prevent genetic diseases - The news at a glanceA new paper in Nature reports the correction of a disease-causing mutation in human embryos. The study is the result of an international collaboration of research centers: the Center for Genome Engineering, within the Institute for Basic Science (IBS, South Korea), Oregon Health and Science University (OHSU, USA), Salk Institute for Biological Studies (USA), BGI-Qingdao and Shenzhen Engineering Laboratory for Innovative Molecular Diagnostics (China). The scientists used the groundbreaking gene-editing tool CRISPR-Cas9 to repair the DNA piece that causes a common genetic heart disease known as hypertrophic cardiomyopathy. This technique would prevent the disease from being inherited by succeeding generations. The experiments on the embryos were conducted in the US following all ethical guidelines. IBS researchers provided CRISPR-Cas9 and analyzed the DNA of the embryos to make sure that the procedure worked correctly. CRISPR-Cas9 combined with in vitro fertilization (IVF) and preimplantation genetic diagnosis (PGD) could be helpful for many other genetic diseases. However, the scientists stated that "genome editing approaches must be further optimized" before moving to clinical trials. "We have succeeded in correcting the mutated gene which causes hypertrophic cardiomyopathy in human embryos with high efficiency and specificity," stresses Jin-Soo KIM, Director of the Center for Genome Engineering, within the Institute for Basic Science (IBS). "Research on human embryos has been a very sensitive subject. The application of this technology to clinical practice in the future requires not only additional research, but also social consensus." Hypertrophic cardiomyopathy: A genetic disease that kills athletesHypertrophic cardiomyopathy is one of more than 10,000 inheritable diseases caused by an error in a single gene. This genetic disease manifests only in adulthood and affects an estimated 1 in 500 people. It can lead to heart failure and sudden death of apparently healthy people. It is well known by sports doctors because training worsens the condition in athletes who suffer from this disease. It is an autosomal dominant genetic disease, meaning that patients with a single copy of the mutant gene are affected and have a 50% chance of transmitting it to their offspring. Current treatments rely mainly on symptomatic relief.
Forty percent of all familial hypertrophic cardiomyopathy is caused by a mutation of the MYBPC3 gene on the 11th chromosome. In this study, the researchers dealt with a mutation characterized by four missing base pairs in the MYBPC3 gene. Reintroducing these four base pairs with CRISPR-Cas9 in the embryo prevents this mutation from appearing in future generations CRISPR-Cas9: Increasing the rate of healthy embryosThe experiment on human embryos was conducted by the OHSU research team in the United States. Researchers worked with healthy egg cells, donated by women, and sperm of a man affected by hypertrophic cardiomyopathy. The IBS team provided CRISPR-Cas9, a genetic tool that has already been shown to eliminate, add or replace pieces of DNA in specific genes. In this case, it allowed the correction of the hypertrophic cardiomyopathy mutation carried in the DNA of the sperm. CRISPR-Cas9 works as a pair of genetic scissors designed to cut the DNA near the position of the mutation. Then, the cut is spontaneously repaired by the cell with different mechanisms: one repairs the DNA without leaving any trace, while the other introduces some unwanted insertions or deletions of a few base pairs near the cutting site.
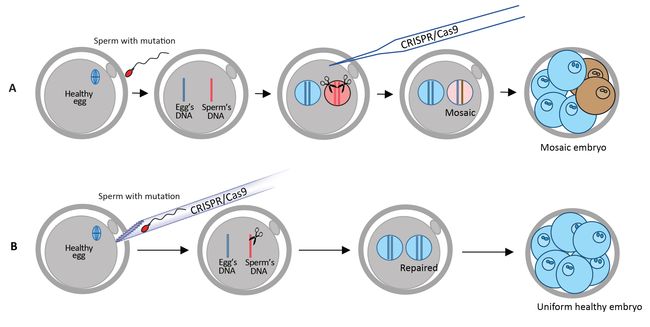
Previous studies have injected CRISPR-Cas9 after IVF, but faced mosaicism problems, characterized by embryos having a mixture of cells with and without the repaired mutation. Mosaicism would lead to organisms with some tissues or organs that bear the mutations and some that do not. In this study, the researchers injected sperm and CRISPR-Cas9 into the egg at the same time to improve the accuracy of the gene correction. Thanks to this strategy, mosaicism did not occur. CRISPR-Cas9 cut the DNA at the correct position in all tested embryos (100%) and 42 out of the 58 embryos (72.4%) did not carry the hypertrophic cardiomyopathy mutation. In other words, this technique increased the probability of inheriting the healthy gene from 50% to 72.4%. Moreover, while doing this research the scientists also discovered that human embryos have an alternative DNA repair system, where the Cas9-induced cuts in the DNA coming from the sperm are repaired using the healthy egg's DNA as a template. In the remaining 27.6% embryos, the cellular cut-repairing mechanism introduced some unwanted insertions or deletions near the cut. Having confirmed that the disease-causing mutation is repaired correctly in human embryos, IBS researchers performed further analysis to make sure that the gene scissors did not cut any other sites of the human genome. The IBS team has previously developed a technique known as Digenome-seq to assess the accuracy of the gene scissors, as off-target cuts and editing mistakes could be a major problem and bring unwanted consequences. Sequencing the whole genome of the embryo did not find any off-target changes. The result was also confirmed with another DNA sequencing technique by researchers working at BGI-Qingdao and Shenzhen Engineering Laboratory for Innovative Molecular Diagnostics.
The ethics of using CRISPR's research on human embryosThe embryo studies were conducted in the USA where this procedure is legal, in adherence to guidelines established by OHSU's Institutional Review Board and additional ad-hoc committees established for scientific and ethical review. The work is also consistent with recommendations issued in February 2017 by the National Academy of Sciences and the National Academy of Medicine joint panel on human genome editing (http://nationalacademies.org/gene-editing/consensus-study/index.htm). Funding for this studyJin-Soo Kim's lab at Seoul National University is supported by the Institute for Basic Science (IBS). Studies conducted at OHSU were supported by OHSU institutional funds, including the Knight Cardiovascular Institute. Work in the laboratory of co-author Juan Carlos Izpisua Belmonte of the Salk Institute was supported by the G. Harold and Leila Y. Mathers Charitable Foundation, the Moxie Foundation, and The Leona M. and Harry B. Helmsley Charitable Trust. Work at BGI was supported by the Shenzhen Municipal Government. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20