주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
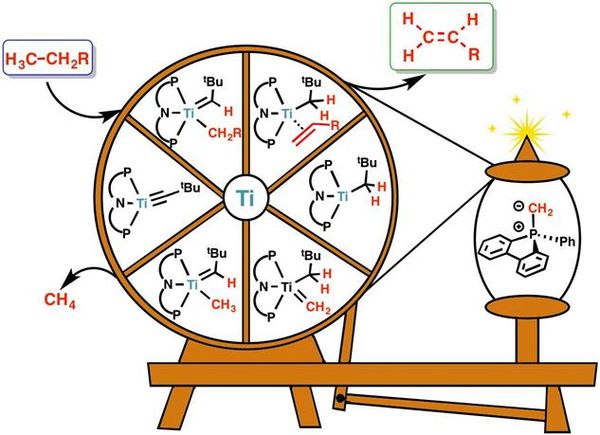
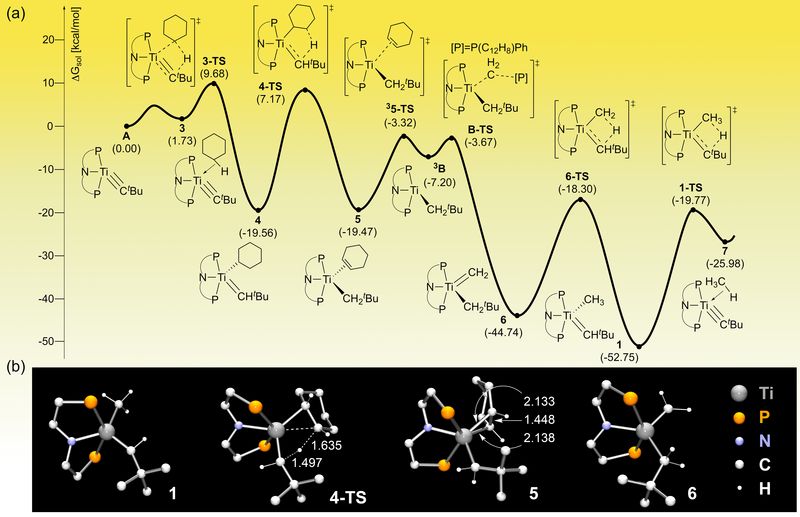
Cheap, Energy-Efficient and Clean Reaction To Make Chemical Feedstock- Combining experimental and computer chemistry, scientists find the conditions to break carbon-hydrogen bonds at low temperature with cheap titanium in place of rare metals - They are all around you! Most plastics, conductive polymers, and even medicines derive from molecules with a double bond between two carbon atoms, C=C. These molecules are called olefins and are mainly produced from fossil fuels through an energy-intensive and polluting process known as steam cracking. It requires temperatures of 800°C and produces the greenhouse gas carbon dioxide. Needless to day, alternatives to this process which could bring environmental and economic benefits are highly sought after. A team of researchers from the Center for Catalytic Hydrocarbon Functionalizations, within the Institute for Basic Science (IBS), in collaboration with Prof. Daniel J. Mindiola from the University of Pennsylvania, accomplished a reaction that was not possible before; they produced olefins with cheap readily available ingredients and at low temperature (75°C). This research outcome, published in Nature Chemistry, paves the way for an efficient use of natural gases to synthesize important chemical products. Natural gases, such as methane and ethane, have strong carbon-hydrogen (C-H) bonds that are difficult to break. The research team managed to transform such unreactive molecules into olefins, the chemical feedstock of a myriad of products we use in our daily life. This type of olefin production method is based on dehydrogenation, that is the removal of hydrogens which leads to the creation of the C=C bond, the mark of olefins. Since the energy required to break the strong C-H bonds is too high, the reaction can be accomplished only with the help of other molecules, called catalysts. Previously, dehydrogenation was possible only with catalysts based on expensive metals, like iridium. The study achieved the cheap production of olefins thanks to a synergistic teamwork between computer and experimental chemists. By simulating the entire chemical process, IBS computer chemists advised their colleagues in the University of Pennsylvania about cheap titanium-based catalyst to test. "We moved from iridium, which is so rare and expensive it is labeled 'the element of the Gods', to an absolutely cheap metal, titanium; an element we are all familiar with as it is broadly used as white pigment for ceramics, paper, and teeth whitener," explains BAIK Mu-Hyun, the leading author from IBS. "The computer simulation predicts the movement of each electron and how molecules are going to interact, so it allowed us to shorten the development time." To summarize, the study showed that making olefins in a cheap, energy efficient way is possible. The reaction can be performed at low temperature and the titanium catalyst can be partially recycled, so it can be used again to dehydrogenate more natural gas. The next challenge of the research team is to make the titanium-based catalyst more efficient.
Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Catalytic Hydrocarbon FunctionalizationsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Suh, William Insang 042-878-8137
- Last Update 2023-11-28 14:20