| Title of announcement | Facilitating speech comprehension in rare inherited hearing loss patients | ||||
|---|---|---|---|---|---|
| Business forms | Y | Expiration date for bidding | |||
| Department in charge | 전체관리자 | Registration Date | 2021-05-25 | Hits | 1183 |
| att. | |||||
|
|
|||||
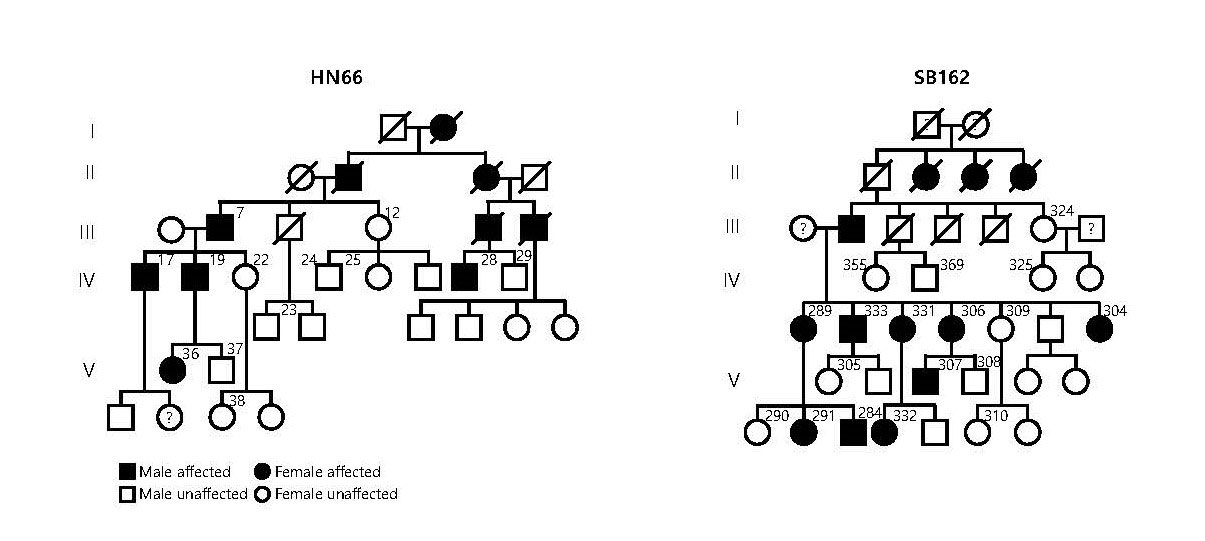
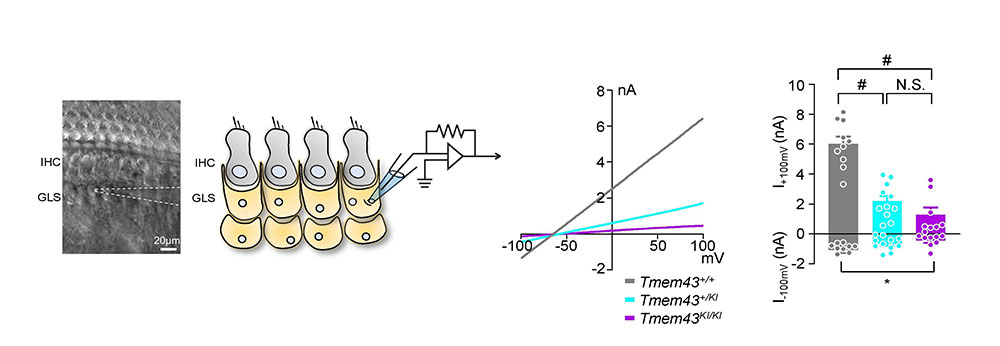
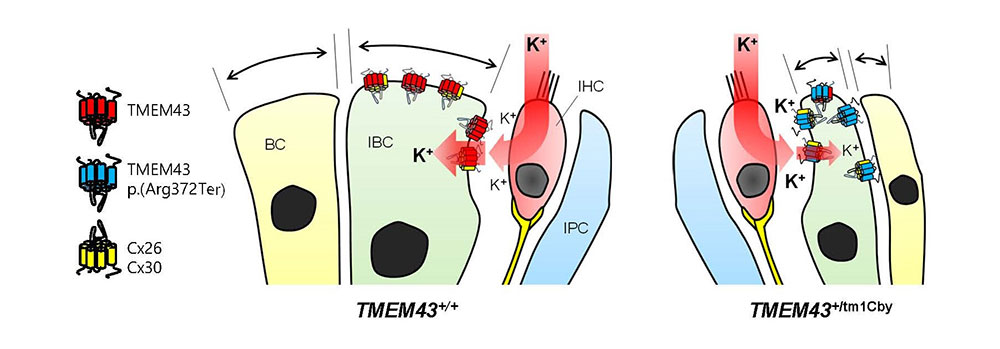
Facilitating speech comprehension in rare inherited hearing loss patientsIBS research team found a novel deafness gene and developed a customized treatment for auditory neuropathy spectrum disorder Hearing loss is a disability that affects approximately 5% of the world’s population. Clinically determining the exact site of the lesion is critical for choosing a proper treatment for hearing loss. For example, the subjects with damage in sound conduction or mild outer hair cell damage would benefit from hearing aids, while those with significant damage to outer or inner hair cells would benefit from the cochlear implant. On the other hand, the subjects with impairments in more central structures such as the cochlear nerve, brainstem, or brain do not benefit from either hearing aids or cochlear implants. However, the role of impairments in cochlear glial cells in hearing loss is not as well known. While it is known that connexin channels in cochlear glial cells play important role in mediating potassium current in the cochlea, the molecular and cellular mechanism of connexin channels and their role in progressive hearing loss has not been studied extensively. Auditory neuropathy spectrum disorder (ANSD) is a confounding auditory disease where the subjects can respond to sound but have difficulties in speech discrimination. Recently, a collaboration of researchers from the Center for Cognition & Sociality (IBS), Seoul National University Bundang Hospital, Mokpo National University, Central South University, and the University of Miami, identified a previously unknown deafness gene that causes this disease. The new gene, TMEM43, is located in chromosome 3 and is mainly expressed in glia-like supporting cells (GLSs) of the cochlea. They identified that the p.(Arg372Ter) variant of TMEM43 was inherited in two prominent Asian families in South Korea and China who are known to suffer from ANSD. In order to study this disease, researchers used a transgenic mouse model with the defective TMEM43-p.(Arg372Ter) gene. Researchers found out that this mouse model exhibited the same progressive hearing loss as the human ANSD subjects. Further examination of these mice revealed that the GLS cell size was smaller in older mice in comparison to that of the control mice. In addition, the TMEM43 protein was shown to interact with connexin channels (Cx26 and Cx30), which are known to regulate K+ conductance in the cochlea. The K+ conductance was significantly impaired in the mutant mouse. Based on the mechanistic insights from the mouse model, researchers performed cochlear implants on 3 human ANSD patients, which resulted in the successful restoration of their speech discrimination. This study is notable in that they identified a novel deafness gene from humans, studied the underlying mechanism of the genetic variant found from human subjects using a mouse model and applied the result back to human patients with successful clinical outcomes. Therefore, this study provides a model platform in which the personalized model of auditory rehabilitation can be determined, highlighting the importance of a precision medicine-based approach. Dr. CHOI Byung Yoon stated that “We are excited to add TMEM43 to the list of genes that cause deafness. It will greatly contribute to accurate diagnosis and customized treatment of deafness with a simple genetic test.” In addition, Director C. Justin Lee mentioned that “This study emphasizes the important role of glial cells in the cochlea. We are positive that further research will give hope to many patients who suffer from this rare form of deafness.” As a future follow-up study, the researchers look forward to recruiting additional subjects with other ANSD gene variants in order to provide further clinical insights into this rare deafness disease. Based on the fact that TMEM43 protein contributes to K+ conductance on cochlear glial cells, researchers plan to continue their study about the role of TMEM43 protein and the ion channels in the brain.
Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||||