| Title of announcement | Using Lasers to See the Shape of Molecules | ||||
|---|---|---|---|---|---|
| Business forms | Expiration date for bidding | ||||
| Department in charge | 전체관리자 | Registration Date | 2015-06-24 | Hits | 5467 |
| att. | |||||
|
|
|||||
|
Using Lasers to See the Shape of Molecules
A Korean research team has created a new technique for resolving the orbits of multiple molecular orbitals, a previously impossible feat
A scientist in a crisp, white lab coat and protective eye goggles sits behind a safety shield, controller in hand. In front of him is a powerful titanium-sapphire laser, aimed at a crystal lens. His thumb gently squeezes the trigger on the controller. There is an imperceivable wisp of gas that is escaping from a nozzle and crossing the laser’s path. Before he can even blink his eye the laser is capable of firing more than a trillion times. On the screen a line of alternating pairs of glowing, amorphous spots appear. For the first time ever, someone has been able to peer down into the molecular level to observe simultaneously in two dimensions.
Professor Hyeok Yun and his team from the Institute for Basic Science (IBS) and Gwangju Institute of Science and Technology (GIST) in Korea have gotten a step closer to fully understanding the complicated relationship of form and motion of molecules.
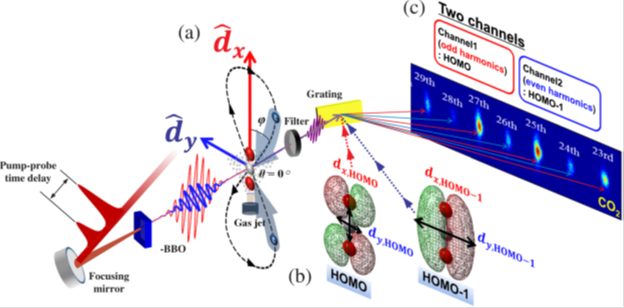
The structure and movement of molecules is not feasible to observe via conventional microscopic methods. In order to get information about molecular shape and the orientation of their orbits, researchers use a process called high harmonic generation (HHG). To do this, a laser pulse tuned to a specific high frequency is directed into a jet of gas of the molecule being studied. When the pulse meets the jet of gas, plasma is generated which emits specific color light. This interaction with the molecule and light generation reveals what is called the highest-occupied molecular orbital (HOMO). The HOMO can be envisioned as the “shape” of the outside molecular orbits. The pulsed laser beam is converted to a high harmonic frequency which reaches the sensor where data from the interaction can be collected. What the researchers see allow them to gather information about the characteristics of molecule’s structure and dynamics. As useful as this technology is, researchers have been limited in what information they can obtain because they have been confined to observing the high harmonic frequency from a single laser pulse on a one-dimensional plane each time.
To gather more information from the molecules during each test, Professor Yu’s team, have devised a method for resolving multiple molecular orbitals by using two-dimensional high-harmonic spectroscopy (HHS). This HHS process involves pulsing a laser at an ultra-fast interval through a polarizing lens which splits the beam in two.
The team focused the laser through a thin crystal which split the beam into two polarized waves traveling in the same direction but now perpendicular to each other. When one beam traveling up and down while the other is moving side to side, the beams are moving orthogonally. When the two beams interacted with the gas sample, they revealed not only the HOMO, but simultaneously the HOMO-1, a lower lying molecular orbit. In the past these two orbits have been difficult to distinguish from one another, because HOMO-1 has been overshadowed by the more energetic HOMO. According to Yun, “In this work, we approached molecules in two dimensions. HOMO-1 can be revealed with relative ease in the orthogonal direction to the molecular axis, while HOMO does it in a parallel direction. Orthogonally polarized two waves enable us to probe both orbitals in two dimensions and to separate signals to different harmonic frequencies. Thus, we could resolve the signals from the two orbitals and could simultaneously obtained information on both orbitals.”
After combining the data collected from each laser pulse the researchers were able to use a clever technique called tomography to piece the two-dimensional images together into a three-dimensional approximation. With the three-dimensional approximation, they were able to discern the shape and relative alignment of the HOMO and HOMO-1 orbitals, something that had never been done before.
There is no loud applause, nobody waiting to congratulate Professor Yun on this achievement.
“The ultimate goal” he says, “is to follow a chemical reaction in its own time scale. It leads us to have direct insight and to understand fundamental mechanism about transformations in molecular scale. We expect this method can be a route or be of help to achieve the goal.” This new method will advance future molecular research by allowing for independent and simultaneous observation of the structures and dynamics of multiple molecular orbitals. It will enable the observation of multi-orbital dynamics during chemical reactions of more complicated molecules.
by Daniel Kopperud # # #
Notes for editors
References
Title of Paper: Resolving Multiple Molecular Orbitals Using Two-Dimensional High-Harmonic Spectroscopy, Physical Review Letters, DOI: dx.doi.org/10.1103/PhysRevLett.114.153901
Authors: Hyeok Yun, 1 Kyung-Min Lee,1 Jae Hee Sung,1,2 Kyung Taec Kim,1,3 Hyung Taek Kim,1,2,* and Chang Hee Nam1,3,† 1Center for Relativistic Laser Science, Institute for Basic Science (IBS), Gwangju 500-712, Republic of Korea 2Advanced Photonics Research Institute, Gwangju Institute of Science and Technology (GIST), Gwangju 500-712, Republic of Korea 3Department of Physics and Photon Science, Gwangju Institute of Science and Technology (GIST), Gwangju 500-712, Republic of Korea
For further information or to request media assistance, please contact: Mr. Shi Bo Shim, Head of Department of Communications, Institute for Basic Science (+82-42-878-8189; sibo@ibs.re.kr) or Ms. Sunny Kim, Department of Communications, Institute for Basic Science (+82-42-878-8135; sunnykim@ibs.re.kr)
About Institute for Basic Science (IBS) The IBS was founded in 2011 by the government of the Republic of Korea. With the sole purpose of driving forward the development of basic science in Korea, IBS will be comprised of a total of 50 research centers in all fields of basic science, including mathematics, physics, chemistry, life science, earth science and interdisciplinary science. IBS has launched 24 research centers as of January 2015. There are one mathematics, eight physics, six chemistry, seven life science, and two interdisciplinary research centers.
|
|||||