주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
|
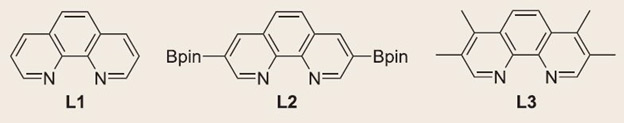
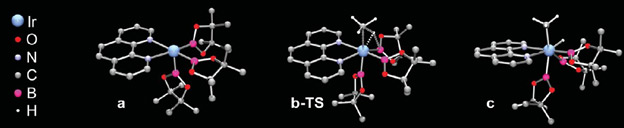
Efficient Methane C-H Bond Activation Achieved for the First Time “This is a really special reaction, one that we all dream about doing.” - Mu-Hyun Baik March 24, 2016 As organic chemistry evolved, techniques for catalyzing hydrocarbons advanced and one by one a method was created for their manipulation. Ethane, propane, butane, pentane—all the alkanes followed a similar pattern and their reactions had predictable results. Only one alkane–methane– refused to follow suit and this confounded chemists for decades. Methane had been just too difficult to work with; its C-H bond could not be manipulated. Using a new hybrid breed of computational and experimental chemistry, an international team of chemists, led by IBS Center for Catalytic Hydrocarbon Functionalizations Associate Director Mu-Hyun Baik, was able to solve a puzzle that has been dubbed a “Holy Grail reaction” and devise a method for catalyzing reactions with methane. Baik believes that while his team’s result is important, the process may have equal value, saying that it may actually be the bigger story. They used a method that is still in its infancy, one that relies as heavily on computer models as experiments in the lab. Baik acknowledges that even a few years ago computers weren’t powerful enough to do this kind of work but thanks to advancements in computing, using them for predictive models is now possible. This newly acquired computing capability allowed Baik’s team to operate using a “very efficient discovery process”. His team’s use of a combined computational and experimental technique is something that has elevated their discoveries far beyond pedestrian trial and error. Instead Baik refers to it as “rational design”, a term usually reserved for discussions of the divine. He was able to model the reactions with the metal catalyst and supporting ligand, (an otherwise inert organic molecule that helps to activate the transition metal catalyst) and after finding success in the virtual setting, tell his colleagues which experiments to pursue.
In the lab, the experimental arm of the team tested a variety of combinations of ligands until they had found the one that worked most efficiently. They used this ligand with different combinations of catalyst and for varied durations to determine the optimum reaction. They found that they were able to generate yields of up to 52%. While this yield was impressive, it may be less significant than the fact that the reaction was done at all. Baik hopes that other researchers will take this knowledge and push the technology further.
Despite the fact that iridium, and the ligands work well together, as does Baik’s method for catalyzing reactions with methane, Baik is ready to scrap everything and refine the process to make it better. Iridium is a rare and extremely expensive metal since it is almost exclusively sourced from meteors. Baik prefers to use something else, like “cobalt, since it is related to iridium, the only difference is that cobalt is dirt cheap and highly abundant.” This paves the way for more chemistry to be done as collaboration between computational and experimental models. Baik’s team has shown that as computers get more sophisticated, computational chemistry will only become a bigger part of deciding what physical experiments to run. Petroleum is quickly becoming a dwindling resource and new methods of creating hydrocarbons are going to be necessary. Being able to manipulate methane means it can be easily converted to liquid methanol and shipped for fuel, which will be a crucial step towards petroleum independence. Besides fuel, petroleum provides the building blocks for things like medicine, fertilizers, and plastics. Baik is certain that “We are all going to have to prepare for a fossil fuel free society.” Luckily for us, he may be the one leading the way. Daniel Kopperud Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Catalytic Hydrocarbon FunctionalizationsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Suh, William Insang 042-878-8137
- Last Update 2023-11-28 14:20