| Title of announcement | From C-H to C-C at Room Temperature | ||||
|---|---|---|---|---|---|
| Business forms | Y | Expiration date for bidding | |||
| Department in charge | 전체관리자 | Registration Date | 2017-12-27 | Hits | 3295 |
| att. | |||||
|
|
|||||
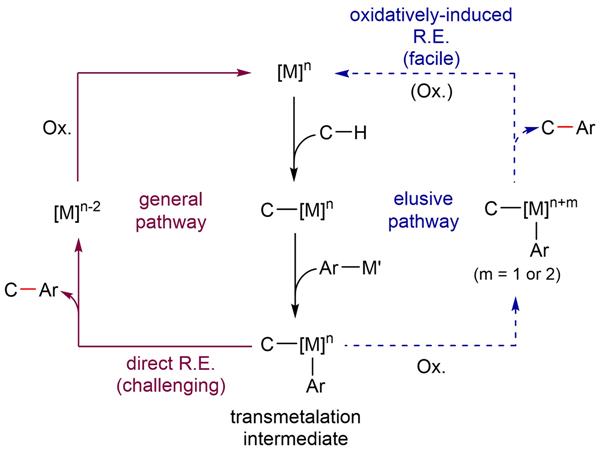
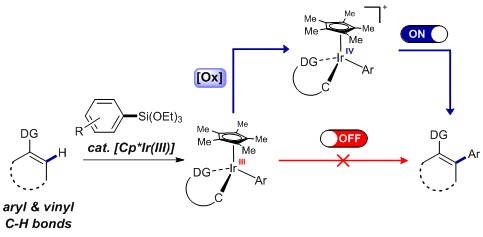
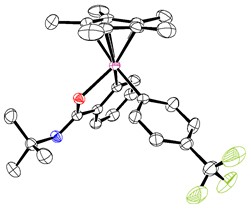
From C-H to C-C at Room Temperature- By oxidizing the iridium center of the reaction intermediate, Carbon-carbon (C-C) bonds make up the skeleton of all organic molecules. However, creating such ubiquitous C-C bonds artificially is still a complicated task. In particular, since several molecules used in medicine, pharmacology and material chemistry contain aryl groups, devising a way to efficiently and selectively introduce this chemical group is a major goal for organic chemists. Currently, most arylation reactions require harsh reaction conditions, including high temperatures and excess additives. Scientists at the Center for Catalytic Hydrocarbon Functionalization, within the Institute for Basic Science (IBS, South Korea), devised a method to selectively introduce aryl groups into C-H bonds at room temperature. Published in Nature Chemistry, the study also clarifies the details of this reaction, which turned out to be different from the conventional idea. In simple terms, the procedure consists of three main steps. Firstly, the iridium catalyst activates the C-H containing substrate. Secondly, the arylsilane attacks the metal, creating an intermediate molecule. The team crystallized such intermediate and demonstrated that oxidizing the iridium center of the intermediate (third step) is beneficial to achieve a low energy arylation reaction. The proposed reaction mechanism was verified with electroparamagnetic resonance, cyclic voltametry and computer simulations. “Developing more efficient and environmetally benign oxidation system is our next goal,” concludes Kwangmin Shin, first author of the study.
Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||||