| Title of announcement | Gel to Fight Rheumatoid Arthritis | ||||
|---|---|---|---|---|---|
| Business forms | Y | Expiration date for bidding | |||
| Department in charge | 전체관리자 | Registration Date | 2017-10-11 | Hits | 3427 |
| att. | |||||
|
|
|||||
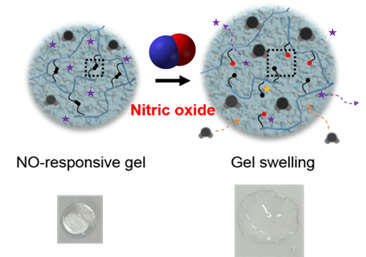
Gel to Fight Rheumatoid Arthritis- IBS scientists developed a potentially therapeutic gel, which activates in the presence of nitric oxide, absorbs excess fluids and delivers drugs - IBS scientists at the Center for Self-Assembly and Complexity, within the Institute for Basic Science (IBS), invented a hydrogel to fight rheumatoid arthritis and other diseases. Published in Advanced Materials, this jelly-like material could be used to absorb extra fluids in swelling joints and release drugs. Rheumatoid arthritis (RA) is a long-term disease that causes inflammation and deformity of the joints, and affects about 1% of the world population. In this autoimmune condition, the body's immune system attacks the soft tissue of the joints, leading to an accumulation of synovial fluid. While this clear fluid lubricates and nourishes the joints, its excess causes swelling and pain. Immune cells at the inflamed joints are the predominant source of nitric oxide (NO), a gas with various physiological functions. “Nitric oxide is like a double-edge sword. It regulates inflammation and protects our body by killing external pathogens. However, when in excess, it is toxic and may cause RA, as well as other autoimmune diseases, cardiovascular diseases, and cancer,” explains the group leader, KIM Won Jong. Current RA treatments are based on anti-inflammatory drugs that relieve pain and inflammation, but IBS scientists tried a more challenging approach by targeting NO itself. NO is a transient gas, which stays in circulation for less than 10 seconds, before binding to other molecules. The research team developed a gel responsive to such fugitive molecules, using acrylamide as a base material and a new crosslinker to keep it in place. Unlike the monomeric form, polymeric acrylamide hydrogel has little toxicity and can contain a large amount of water. In addition, the cross-linking agent (NOCCL) forms bridges between the acrylamide molecules creating a net, which can trap drug molecules inside. When NO cleaves the NOCCL bridges, the gel changes its structure, frees the drug and absorbs new liquid.
Although this is a preliminary study, the team confirmed that NOCCL crosslinking agent can selectively and sensitively react with NO. The scientists are now working on a nano-sized hydrogel in a RA mouse model. Moreover, these type of hydrogels could be useful for other diseases characterized by overexpression of NO, or maybe even as environmental sensors, since NO is also a polluting gas emitted in vehicle exhaust. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||||