주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Proper synaptic joint will get you good night’s sleep | ||
|---|---|---|---|
| Embargo date | 2020-05-14 14:11 | Hits | 661 |
| Research Center |
Center for Synaptic Brain Dysfunctions |
||
| Press release | |||
| att. | |||
Proper synaptic joint will get you good night’s sleepSynaptic adhesion molecule PTPδ regulates synaptic development and sleep behavior in mice Plenty of theories, but nobody knows exactly why we sleep. One thing we know for sure: sleepless nights will not help you make it through the day. There have been many studies exploring how sleep works in the brain and what its purposes are. However, how the synapse – the fundamental building block of the brain contributing to the formation of neuronal circuits – works in relation to sleep has been poorly understood. A research team led by Director KIM Eunjoon of the Center for Synaptic Brain Dysfunctions within the Institute for Basic Science (IBS, South Korea), has reported in vivo findings that a presynaptic cell adhesion molecule named PTPδ is crucial for the development of synapses in the developing brain. Thus, the genetic deletion of PTPδ leads to disruptions in structural, functional, and biochemical compositions in the brain of the afflicted mice, resulting in changes in innate behaviors, such as hyper-locomotor activity, increased anxiety, and decreased sleep. The human brain contains billions of neurons which communicate with each other via synapses. The signals one neuron sends to another via the release of neurotransmitters at presynaptic sites are sensed by neurotransmitter receptors located at postsynaptic sites of other neurons. In turn, networks of neurons determine the expression of all behaviors and bodily functions controlled by the brain. At the core of all these comprehensive networks for brain functions are the synaptic cell adhesion molecules (CAMs). CAMs are essential for regulating the development of synapses by bridging the pre- and postsynaptic sides. To ensure normal development of neuronal circuits and brain functions, it is critical to make appropriate paring of pre- and postsynaptic CAMs, among many different kinds, during the development of young neurons.
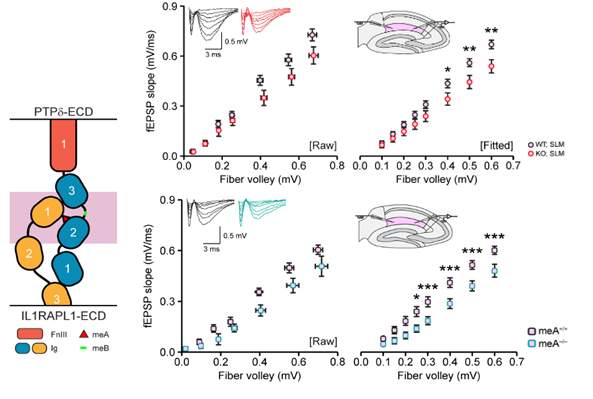
The diagram on the left depicts the interaction of presynaptic PTPδ with postsynaptic IL1RAPL1 in the synapse, with the crucial meA structure shown as the red triangle. The graphs on the right show decreases in synaptic transmission in the hippocampus of mice with PTPδ deletion (top) and PTPδ-meA deletion (bottom). The research team focused on finding specific roles of PTPδ, as it was only suspected of playing a key role in synaptic formation. They developed a fluorescent molecule-tagged version of PTPδ, whose expression in the brain allowed precise and clear-cut visualization of the natural location of PTPδ across the brain and down to the position within the synapse with nanometer scale precision. They also adopted conditional deletions of PTPδ to pinpoint the key joint component in synaptic formation. Crucially, the team has demonstrated that disruptions of presynaptic PTPδ interaction with its postsynaptic binding partner IL1RAPL1 cause all key changes in innate behaviors as shown in Figures 2 and 3.
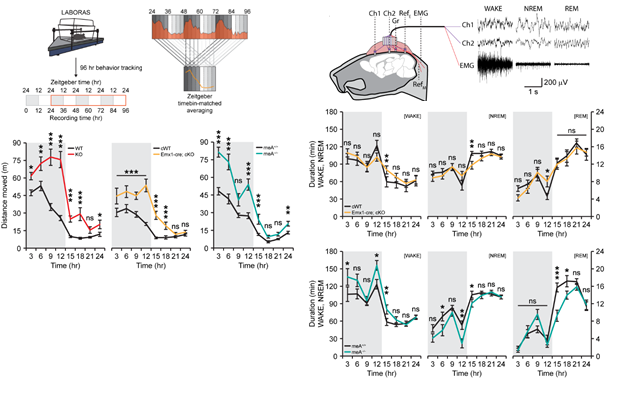
The graphs on the left show the presence of hyperactivity throughout 24 hours of mice with PTPδ whole deletion (red), PTPδ-excitatory neuron deletion (yellow), and PTPδ-meA deletion (aquamarine) compared to control mice (black). The graphs on the right show decreases of non-REM sleep, representing deep sleep with delta waves, in both the PTPδ-excitatory neuron deletion (yellow) and PTPδ-meA deletion (aquamarine) mice.
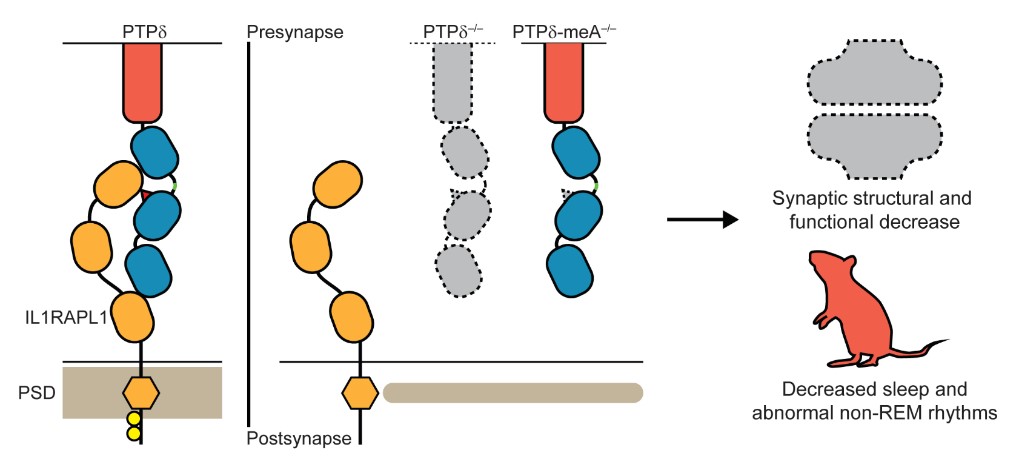
On the left, normal trans-synaptic interaction of presynaptic PTPδ and postsynaptic IL1-RAPL1 in the synapse, which critically requires the meA in PTPδ (red triangle). In the middle, disruption of the trans-synaptic PTPδ-IL1RAPL1 interaction through the loss of the whole PTPδ or of the meA. On the right, the loss of the trans-synaptic PTPδ-IL1RAPL1 interaction leads to the loss of synaptic structure and function along with altered brain functions manifested by decreased sleep and abnormal sleep rhythms as seen in delta waves during non-REM deep sleep. It has been previously shown that the trans-synaptic interaction (the interaction that spans across the synapse from one neuron to the next) depends on a six amino acid-long short peptide sequence (known as meA) within the PTPδ protein. The research team used this knowledge to specifically block the PTPδ-IL1RAPL1 interaction. When the key meA sequence is deleted, leaving the rest of PTPδ functional and intact, IL1RAPL1 is unable bind to PTPδ and therefore unable to stay on the postsynaptic side of a synapse. This leads to a significant decrease in the number of synapses across the brain, disrupting the intricate neuronal circuits that form and determine behavior output such as sleep. They also selectively deleted PTPδ on excitatory neurons and confirmed the hypothesis that the synapse-to-behavior disruptions were indeed localized to excitatory neurons. The research team also showed that when IL1RAPL1 is unable to bind to PTPδ across the synapse, the chemical composition of IL1RAPL1 itself changes, with near-complete block of a process called tyrosine phosphorylation in its amino acid sequence. This change in the innate property of IL1RAPL1 is accompanied by exclusion of IL1RAPL1 from the synapse. Because IL1RAPL1 is crucial for the maturation of the synapse, this exclusion leads to structural and functional decreases of the synapse. Disruption of the brain at such a fundamental level leads to the increase in anxiety and decrease in sleep seen in the PTPδ-mutant mice. There have been several studies that linked synaptic CAMs with abnormal mouse behaviors. Notably, this study offers the first comprehensive overview of how CAMs are linked with sleep, a fundamental brain function. In addition, no previous studies have associated a specific pair of pre- and postsynaptic CAMs with sleep or sleep or sleep-related behaviors such as hyperactivity and anxiety. As mutations in the gene coding for PTPδ are associated with numerous psychiatric disorders, such as schizophrenia, attention deficit hyperactivity disorder (ADHD), and restless leg syndrome (a type of sleep disorder) with each of these disorders affecting 1% to 5% of the general population worldwide, this study shines light on how changes in PTPδ may lead to symptoms reminiscent of the aforementioned disorders. The development of the brain during the embryonic and early postnatal stages are crucial for the proper expression of behavior and brain-related functions. This study shows that even the singular disruption of the PTPδ-IL1RAPL1 interaction, among many possible pairs of pre- and postsynaptic CAMs, has consequences that last the entire lifetime of the organism, such as a permanent reduction of sleep. Given that sleep is essential to survival, the disruption of a single binding pair at the synapse can have such far-reaching consequences speaks of the importance of this discovery, which will further our understanding of how the basic building blocks of the brain influences complex behaviors such as sleep. Dahee Carol Kim Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20